Method discovered to remove damaging amyloid plaques found in Alzheimer’s disease
December 23, 2016
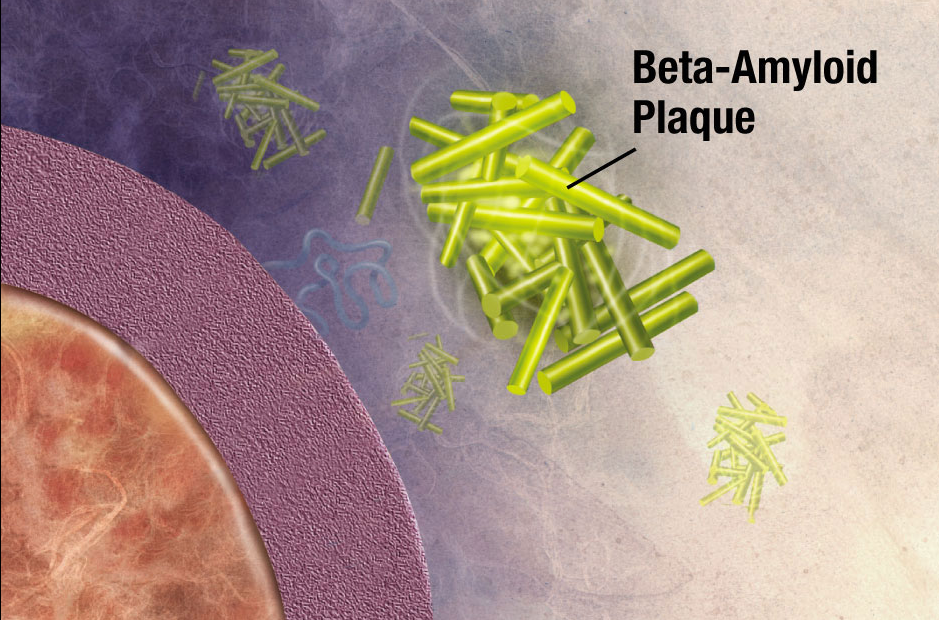
Illustration of formation of beta-amyloid plaque. Enzymes act on the APP (amyloid precursor protein) and cut it into fragments. The beta-amyloid fragment is crucial in the formation of senile plaques in Alzheimer’s disease. (credit: National Institute on Aging/NIH)
German scientists have discovered a strategy for removing amyloid plaques — newly forming clumps in a brain with Alzheimer’s disease that are created by misfolded proteins that clump together and damage nerve cells.
The scientists from the German Center for Neurodegenerative Diseases (DZNE) in Munich and the Ludwig Maximilians University (LMU) Munich took aged microglia cells (the scavenger cells of the brain’s immune system) from a mouse model of Alzheimer’s disease and co-cultured them with microglia tissue from younger mouse brains. Surprisingly, within a few days, they found that the older amyloid plaques started to rejuvenate, resume cell division, and clear the brain from plaques by engulfing them. The amyloid plaque clearance potential of aged Alzheimer’s disease microglia could be fully restored, despite the continuous loss of neurons and astrocytes.
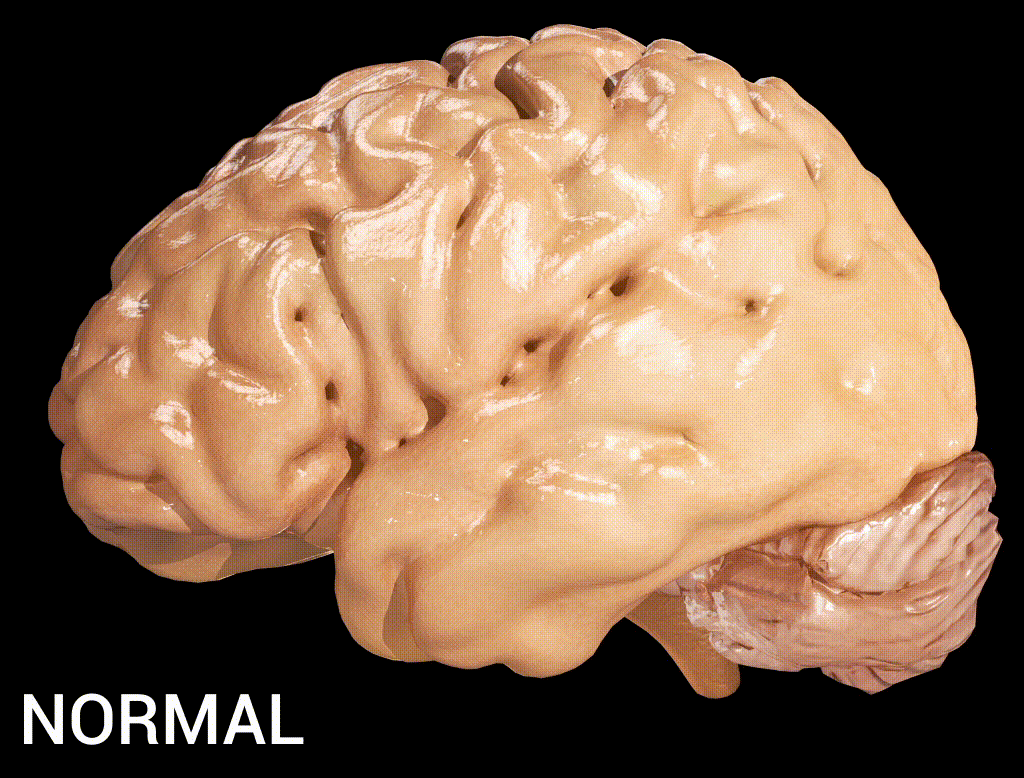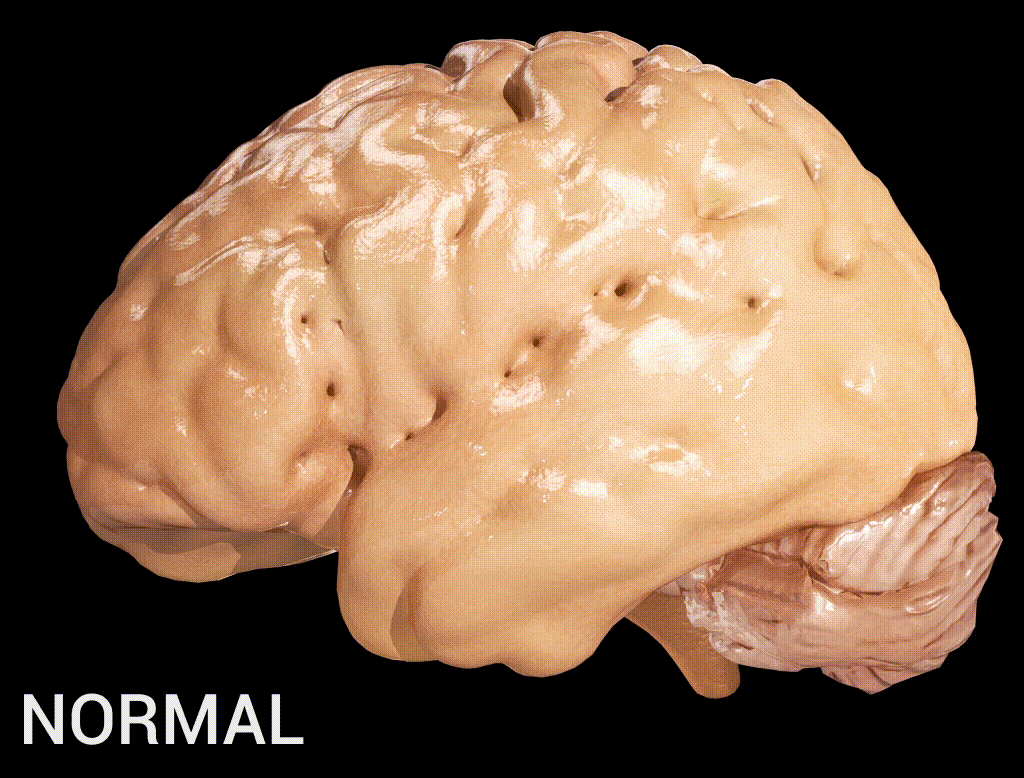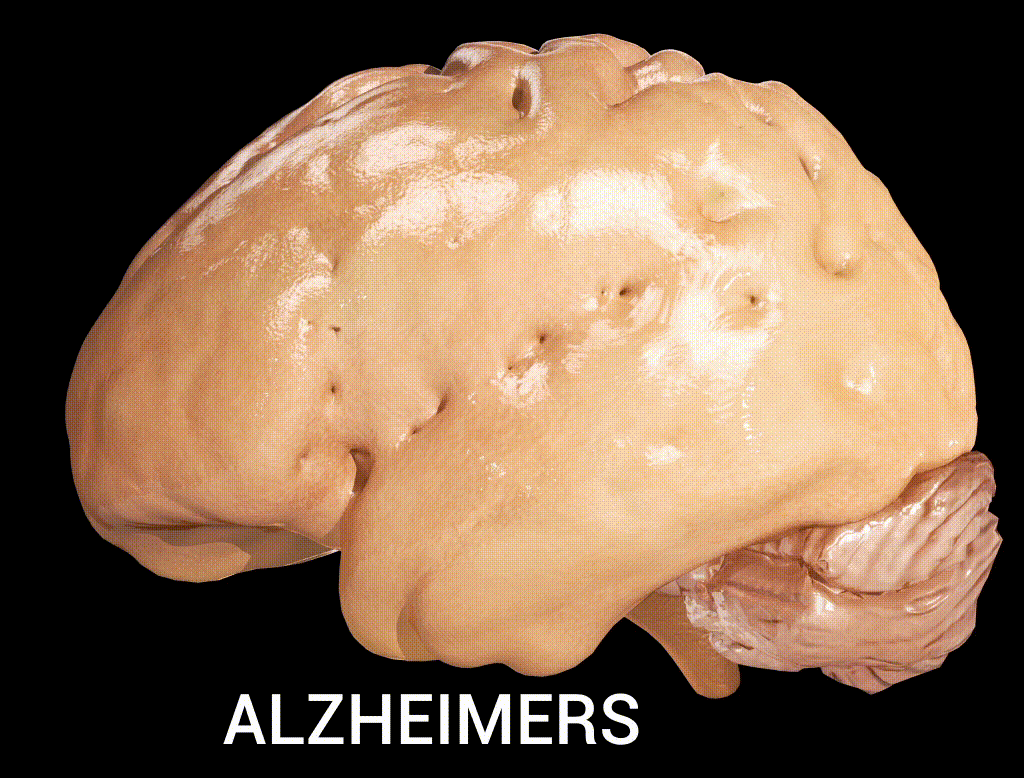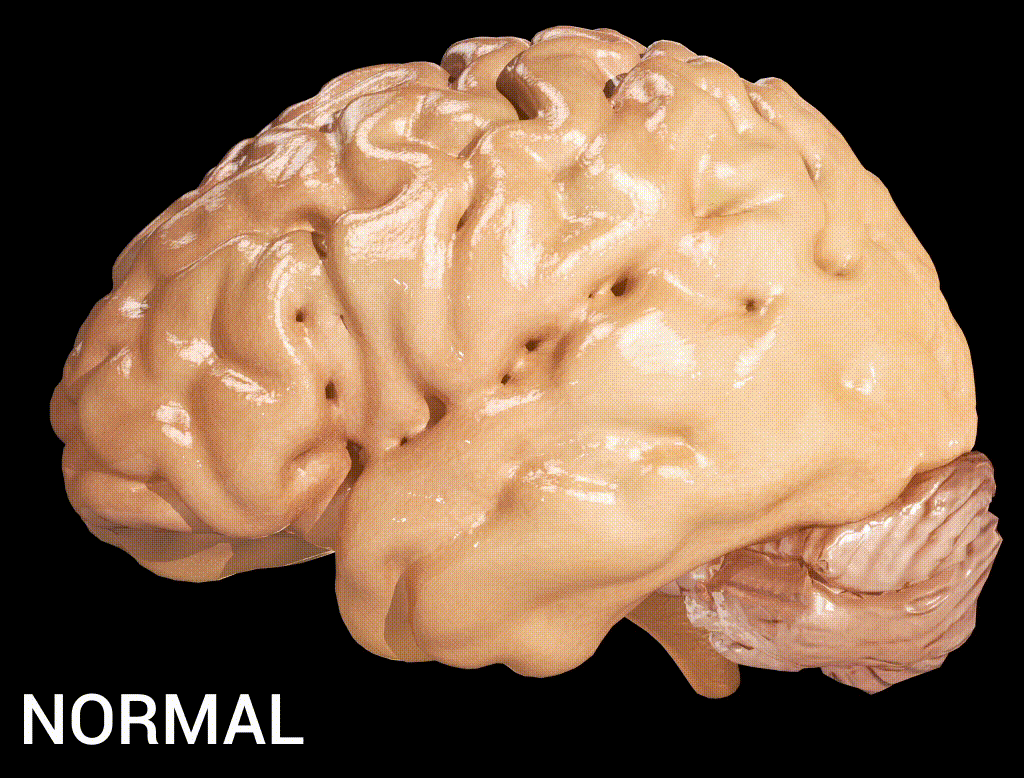
Cortical atrophy in Alzheimer’s Disease, associated with loss of gyri and sulci in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus (credit: Doctor Jana/CC via Wikipedia)
They also found that the young microglia cells were secreting factors that helped the old microglia rejuvenate — especially a protein called “granulocyte-macrophage colony stimulating factor” (GM-CSF)* — which alone could do the job. Depletion of either old or young microglial cells prevented amyloid plaque clearance, indicating a synergistic effect of both populations.

Click the image to view a DZNE video that describes the role of dysfunctional proteins in creating amyloid plaque in early Alzheimer’s disease, starting at 2:25. (credit: DZNE)
GM-CSF has previously been reported to reduce plaques and improve cognition in a mouse model of Alzheimer’s disease, but it’s not yet known if GM-CSF could potentially work as a new drug for Alzheimer’s disease in humans. Also, microglia secrete small proteins that induce inflammatory reactions and may harm neurons, the researchers say.
The researchers suggest that the new model system can be explored further to search for additional factors that enhance the clearance of amyloid plaques.
The work was published this week in The EMBO Journal.
National Institute on Aging | Inside the Brain: Unraveling the Mystery of Alzheimer’s Disease
* GM-CSF is manufactured using recombinant DNA technology. It is marketed as a protein therapeutic alled molgramostim or, when the protein is expressed in yeast cells, sargramostim. It is used as a medication to stimulate the production of white blood cells and thus prevent neutropenia (loss of neutrophils, a type of white blood cell) following chemotherapy. (Patients with neutropenia are more susceptible to bacterial infections and, without prompt medical attention, the condition may become life-threatening.)
Abstract of Young microglia restore amyloid plaque clearance of aged microglia
Alzheimer′s disease (AD) is characterized by deposition of amyloid plaques, neurofibrillary tangles, and neuroinflammation. In order to study microglial contribution to amyloid plaque phagocytosis, we developed a novel ex vivo model by co‐culturing organotypic brain slices from up to 20‐month‐old, amyloid‐bearing AD mouse model (APPPS1) and young, neonatal wild‐type (WT) mice. Surprisingly, co‐culturing resulted in proliferation, recruitment, and clustering of old microglial cells around amyloid plaques and clearance of the plaque halo. Depletion of either old or young microglial cells prevented amyloid plaque clearance, indicating a synergistic effect of both populations. Exposing old microglial cells to conditioned media of young microglia or addition of granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) was sufficient to induce microglial proliferation and reduce amyloid plaque size. Our data suggest that microglial dysfunction in AD may be reversible and their phagocytic ability can be modulated to limit amyloid accumulation. This novel ex vivo model provides a valuable system for identification, screening, and testing of compounds aimed to therapeutically reinforce microglial phagocytosis.