Mimicking atherosclerosis with blood cells on a microchip
February 7, 2014
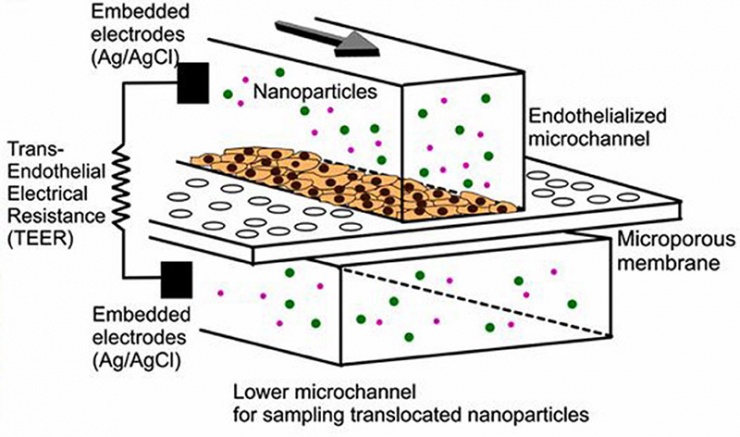
Schematic of a microfluidic device with two-layer microfluidic channels separated by a porous membrane on which blood-vessel endothelial cells are grown (credit: YongTae Kim/PNAS)
Georgia Institute of Technology scientists have engineered a microchip coated with blood vessel cells. The objective: learn more about the conditions under which nanoparticles accumulate in the plaque-filled arteries of patients with atherosclerosis, the underlying cause of myocardial infarction and stroke.
They coated microchips with a thin layer of endothelial cells, which make up the interior surface of blood vessels.
In healthy blood vessels, endothelial cells act as a barrier to keep foreign objects out of the bloodstream. But at sites prone to atherosclerosis, the endothelial barrier breaks down, allowing things to move in and out of arteries that shouldn’t.
Mimicking atherosclerosis
The microchips allow nanoparticles to cross the endothelial cell layer on the microchip under conditions that mimic the permeable layer in atherosclerosis. The results correlated well with nanoparticle accumulation in the arteries of an animal model with atherosclerosis, demonstrating the device’s capability to help screen nanoparticles and optimize their design.
“It’s a simple model — a microchip, not cell culture dish — which means that a simple endothelialized microchip with microelectrodes can show some yet important prediction of what’s happening in a large animal model,” said YongTae (Tony) Kim, an assistant professor in bioengineering in the George W. Woodruff School of Mechanical Engineering at the Georgia Institute of Technology.
The researchers hope that their microchip can accelerate the nanomedicine development process by better predicting therapeutic nanoparticles’ performance in larger animal models, such as rabbits. Such a complimentary model would save time and money and require fewer animals.
Bypassing the 15-year FDA roadblock for nanomedicine platforms
Few nanoparticle-based drug delivery systems, compared to proposed studies, have been approved by the U.S. Food and Drug Administration, Kim said. The entire process developing one nanomedicine platform can take 15 years to go from idea to synthesis to testing in vitro to testing in vivo to approval.
“That’s a frustrating process,” Kim said. “Often what works in cell culture dishes doesn’t work in animal models.”
To help speed up nanomedicine research by improving the predictive capabilities of in vitro testing, Kim and colleagues designed their microchip to mimic what’s going on in the body better than what is currently possible through routine cell culture.
“In the future, we can make microchips that are much more similar to what’s going on in animal models, or even human beings, compared to the conventional cell culture dish studies,” Kim said.
On their microchip, scientists can control the permeability of the endothelial cell layer by altering the rate of blood flow across the cells or by introducing a chemical that is released by the body during inflammation. The researchers discovered that the permeability of the cells on the microchip correlated well with the permeability of microvessels in a large animal model of atherosclerosis.
The microchips allows for precise control of the mechanical and chemical environment around the living cells. By using the microchip, the researchers can create physiologically relevant conditions to cells by altering the rate of blood flow across the cells or by introducing a chemical that is released by the body during inflammation.
Kim said that while this microchip-based system offers better predictability than current cell culture experiments, it won’t replace the need for the animal studies, which provide a relatively more complete picture of how well a particular nanomedicine might work in humans.
“This is better than an in vitro dish experiment, but it’s not going to perfectly replicate what’s going on inside the body in near future,” Kim said. “It will help make this whole process faster and save a number of animals.”
The research is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH). The team includes researchers at the David H. Koch Institute for Integrative Cancer Research at MIT, the Icahn School of Medicine at Mount Sinai, the Academic Medical Center in Amsterdam, Kyushu Institute of Technology in Japan, and the Boston University School of Medicine and Harvard Medical School.
Abstract of Proceedings of the National Academy of Sciences paper
Therapeutic and diagnostic nanomaterials are being intensely studied for several diseases, including cancer and atherosclerosis. However, the exact mechanism by which nanomedicines accumulate at targeted sites remains a topic of investigation, especially in the context of atherosclerotic disease. Models to accurately predict transvascular permeation of nanomedicines are needed to aid in design optimization. Here we show that an endothelialized microchip with controllable permeability can be used to probe nanoparticle translocation across an endothelial cell layer. To validate our in vitro model, we studied nanoparticle translocation in an in vivo rabbit model of atherosclerosis using a variety of preclinical and clinical imaging methods. Our results reveal that the translocation of lipid–polymer hybrid nanoparticles across the atherosclerotic endothelium is dependent on microvascular permeability. These results were mimicked with our microfluidic chip, demonstrating the potential utility of the model system.