MIT uses forests of carbon nanotubes with antibodies to capture hard-to-detect molecules
December 22, 2015
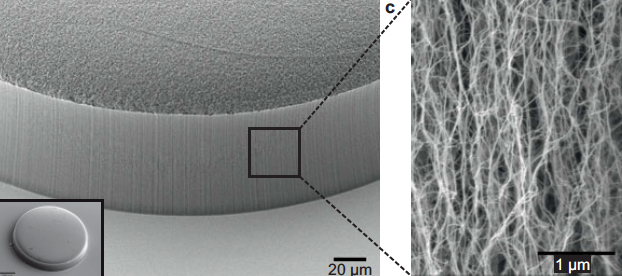
Scanning electron microscope image of carbon nanotubes showing textured porosity (credit: Allison L. Yost et al./Microsystems & Nanoengineering)
Engineers at MIT have devised a new technique for trapping hard-to-detect molecules, using forests of coated carbon nanotubes.
The team modified a simple microfluidic channel with an array of vertically aligned carbon nanotubes — rolled lattices of carbon atoms that resemble tiny tubes of chicken wire.
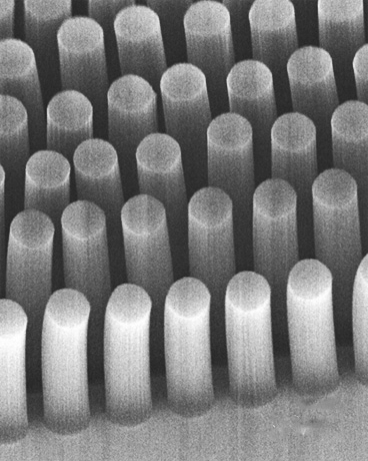
Carbon-nanotube posts can trap cancer and other cells as they flow through a microfluidic device (credit: Brian Wardle)
The researchers had previously devised a method for standing carbon nanotubes on their ends, like trees in a forest (see “Trapping cancer cells with carbon nanotubes“). This 3-D array of permeable carbon nanotubes allows fluid to flow through a microfluidic device.
Now, in a study published this week in the Journal of Microengineering and Nanotechnology, the researchers have given the nanotube array the additional ability to trap specific particles. To do this, the team coated the array, layer by layer, with polymers of alternating electric charge.
Depending on the number of layers deposited, the researchers can create thicker or thinner nanotubes and thereby tailor the porosity of the forest to capture larger or smaller particles of interest. The nanotubes’ polymer coating can also be chemically manipulated to bind specific bioparticles flowing through the forest.
The combination of carbon nanotubes and multilayer coatings may help finely tune microfluidic devices to capture extremely small and rare particles, such as certain viruses and proteins, says Brian Wardle, professor of aeronautics and astronautics at MIT.
“There are smaller bioparticles that contain very rich amounts of information that we don’t currently have the ability to access in point-of-care [medical testing] devices like microfluidic chips,” says Wardle, who is a co-author on the paper. “Carbon nanotube arrays could actually be a platform that could target that size of bioparticle.”
What’s more, Wardle says, a three-dimensional forest of carbon nanotubes would provide much more surface area on which target molecules may interact, compared with the two-dimensional surfaces in conventional microfluidics.
Capturing specific particles of interest
To test this idea, the researchers used an established technique to treat the surface of the nanotubes with antibodies that bind to prostate specific antigen (PSA), a common experimental target.
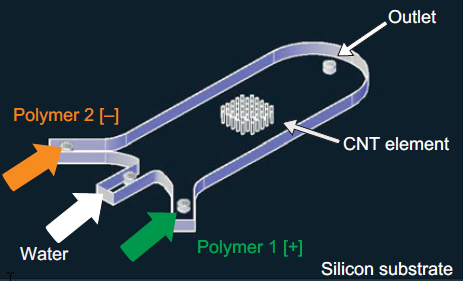
A 3-D array of carbon nanotubes on a microfluidic device coated with successive layers of alternately charged polymer solutions (credit: Allison L. Yost et al./Microsystems & Nanoengineering)
The team integrated a 3-D array of carbon nanotubes into a microfluidic device by using chemical vapor deposition and photolithography to grow and pattern carbon nanotubes onto silicon wafers. They then grouped the nanotubes into a cylinder-shaped forest, measuring about 50 micrometers tall and 1 millimeter wide, and centered the array within a 3 millimeter-wide, 7-millimeter long microfluidic channel.
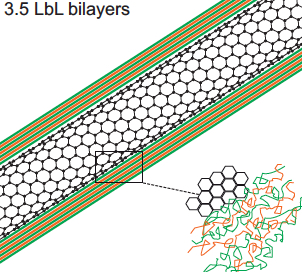
Polyelectrolyte multilayer (PEM) film deposition on carbon-nanotube surface (credit: Allison L. Yost et al./Microsystems & Nanoengineering)
The researchers coated the nanotubes in successive layers of alternately charged polymer solutions to create distinct, binding layers around each nanotube. To do so, they flowed each solution through the channel and found they were able to create a more uniform coating with a gap between the top of the nanotube forest and the roof of the channel. Such a gap allowed solutions to flow over, then down into the forest, coating each individual nanotube.
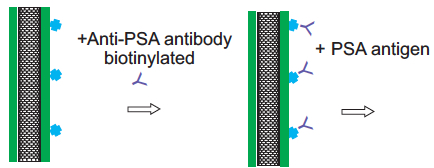
Carbon nanotube treated with antibodies for PSA capture (credit: Allison L. Yost et al./Microsystems & Nanoengineering)
After coating the nanotube array in layers of polymer solution, the researchers demonstrated that the array could be primed to detect a given molecule by treating it with antibodies that typically bind to prostate specific antigen (PSA). They pumped in a solution containing small amounts of PSA and found that the array captured the antigen effectively, throughout the forest, rather than just on the outer surface of a typical microfluidic element.
The polymer-coated arrays captured 40 percent more antigens, compared with arrays lacking the polymer coating.
Wardle says that the nanotube array is extremely versatile. The carbon nanotubes can be manipulated mechanically, electrically, and optically and the polymer coatings can be chemically altered to capture a wide range of particles. He says an immediate target may be biomarkers called exosomes, which are less than 100 nanometers wide and can be important signals of a disease’s progression.
“This type of device actually has all the characteristics and functionality that would allow you to go after bioparticles like exosomes and things that really truly are nanometer scale,” he noted.
This research was funded in part by the National Science Foundation.
Abstract of Layer-by-layer functionalized nanotube arrays: A versatile microfluidic platform for biodetection
We demonstrate the layer-by-layer (LbL) assembly of polyelectrolyte multilayers (PEM) on three-dimensional nanofiber scaffolds. High porosity (99%) aligned carbon nanotube (CNT) arrays are photolithographically patterned into elements that act as textured scaffolds for the creation of functionally coated (nano)porous materials. Nanometer-scale bilayers of poly(allylamine hydrochloride)/poly(styrene sulfonate) (PAH/SPS) are formed conformally on the individual nanotubes by repeated deposition from aqueous solution in microfluidic channels. Computational and experimental results show that the LbL deposition is dominated by the diffusive transport of the polymeric constituents, and we use this understanding to demonstrate spatial tailoring on the patterned nanoporous elements. A proof-of-principle application, microfluidic bioparticle capture using N-hydroxysuccinimide-biotin binding for the isolation of prostate-specific antigen (PSA), is demonstrated.