Nanotech for tissue regeneration
April 18, 2011
Researchers have developed new nanotechnologies for wound healing and blood-vessel tissue, and have determined how ribosomes insert a growing protein into a cellular membrane.
Nanofiber spheres for wound healing
Scientists at the University of Michigan have made star-shaped, biodegradable polymers that can self-assemble into hollow, nanofiber spheres that biodegrade when injected with cells into wounds, while the cells live on to form new tissue.
The nanofibrous hollow spheres are combined with cells and then injected into the wound. The nanofiber spheres are slightly bigger than the cells they carry.
The cells start growing easily in the wound because the nanofiber spheres provide an environment in which the cells naturally thrive.
During testing, the nanofiber repair group grew as much as three to four times more tissue than the control group.
Regenerating blood supply
Researchers at The University of Western Ontario have discovered a strategy for stimulating the formation of highly functional new blood vessels in tissues that are starved of oxygen.
The researchers developed a strategy in which fibroblast growth factor 9 (FGF9) is delivered at the same time that the body is making its own effort at forming new blood vessels in vulnerable or damaged tissue.
The result is that an otherwise unsuccessful attempt at regenerating a blood supply becomes a successful one.
How ribosomes insert membranes into cells
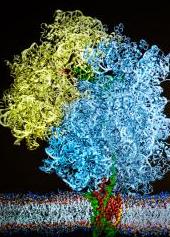
Cryogenic electron microscope images were used to construct an atom-by-atom model of the system that threads a growing protein into a cellular membrane (credit: L. Brian Stauffer)
Computational theoretical scientists at the University of Illinois and experimental scientists at University of Munich have provided the first detailed atom-by-atom view of the elaborate chemical and mechanical interactions that allow the ribosome to insert a growing protein into a cellular membrane.
The first study used cryo-electron microscopy to image one moment in the insertion process. The researchers were able to get a picture of how the ribosome, membrane, membrane channel, and newly forming protein come together to complete the insertion process.
They found that regions of the membrane channel actually reach into the ribosome to help funnel the emerging protein into the channel. Depending on the type of protein being built, the channel will thread it all the way through the membrane to secrete it or open a “side door” that directs the growing protein into the interior of the membrane.
In the second study, the researchers found that proteins get inserted into the membrane in two stages. First, the ribosome “pushes” the growing protein into the membrane channel, and then, in a second step, the protein enters the membrane.
Ref.: The University of Michigan work is scheduled for advanced online publication in Nature Materials
Ref.: Klaus Schulten et al., Cryo-EM structure of the ribosome-SecYE complex in the membrane environment, Nature Structural & Molecular Biology, 2011
Ref.: Klaus Schulten et al., Free-energy cost for translocon-assisted insertion of membrane proteins, PNAS, February 11, 2011
Ref.: J. Geoffrey Pickering et al., Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells, Nature Biotechnology, April 17, 2011