Nanotherapeutic delivers clot-busting drugs directly to obstructed blood vessels
July 12, 2012
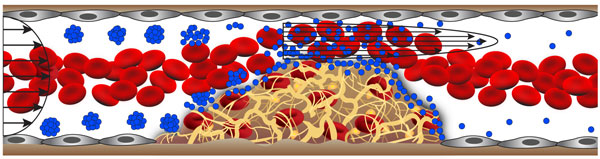
The shear-activated nanotherapeutic breaks apart and releases its drug when it encounters regions of vascular narrowing (credit: Wyss Institute)
A novel biomimetic strategy that delivers life-saving nanotherapeutics directly to obstructed blood vessels, dissolving blood clots before they cause serious damage or even death has been developed by researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University.
This new approach enables thrombus dissolution while using only a fraction of the drug dose normally required, thereby minimizing bleeding side effects that currently limit widespread use of clot-busting drugs.
The research findings have significant implications for treating major causes of death, such as heart attack, stroke and pulmonary embolism, that are caused by acute vascular blockage by blood thrombi.
Background
Disruption of normal blood flow to the heart, lung, and brain due to thrombosis is one of the leading causes of death and long-term adult disability in the developing world. Today, patients with pulmonary embolism, strokes, heart attacks and other types of acute thrombosis leading to near-complete vascular occlusion, are most frequently treated in an acute care hospital setting using systemic dosages of powerful clot-dissolving drugs.
Because these drugs can cause severe and often fatal bleeding as they circulate freely throughout the body, the size of the dosage given to any patient is limited because efficacy must be balanced against risk.
How the targeted vascular nanotherapeutic works
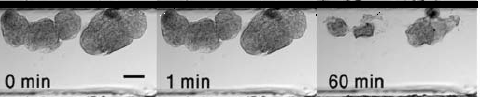
Artificial microemboli (~ 250 μm) in a microfluidic channel before (0 min) and 1 or 60 min after injection of shear-activated nanotherapeutics coated with tPA (50 ng/ml) showing progressive lysis of the clots over time (credit: Wyss Institute)
The inspiration for the targeted vascular nanotherapeutic approach came from the way in which normal blood platelets rapidly adhere to the lining of narrowed vessels, contributing to the development of atherosclerotic plaques. When vessels narrow, high shear stresses provide a physical cue for circulating platelets to stick to the vessel wall selectively in these regions.
The vascular nanotherapeutic is similarly about the size of a platelet, but it is an aggregate of biodegradable nanoparticles that have been coated with the clot-busting drug, tissue plasminogen activator (tPA).
Much like when a wet ball of sand breaks up into individual grains when it is sheared between two hands, the aggregates selectively dissociate and release tPA-coated nanoparticles that bind to clots and degrade them when they sense high shear stress in regions of vascular narrowing, such as caused by blood clot formation.
The new shear-activated nanotherapeutic has the potential to overcome these efficacy limitations. By targeting and concentrating drug at the precise site of the blood vessel obstruction, the Wyss team has been able to achieve improved survival in mice with occluded lung vessels with less than 1/50th of the normal therapeutic dose, which should translate into fewer side effects and greater safety.
This raises the possibility that, in the future, an emergency technician might be able immediately administer this nanotherapeutic to anyone suspected of having a life-threatening blood clot in a vital organ before the patient even reached the hospital.