New antimicrobial peptide kills strains resistant to existing antibiotics
November 4, 2016
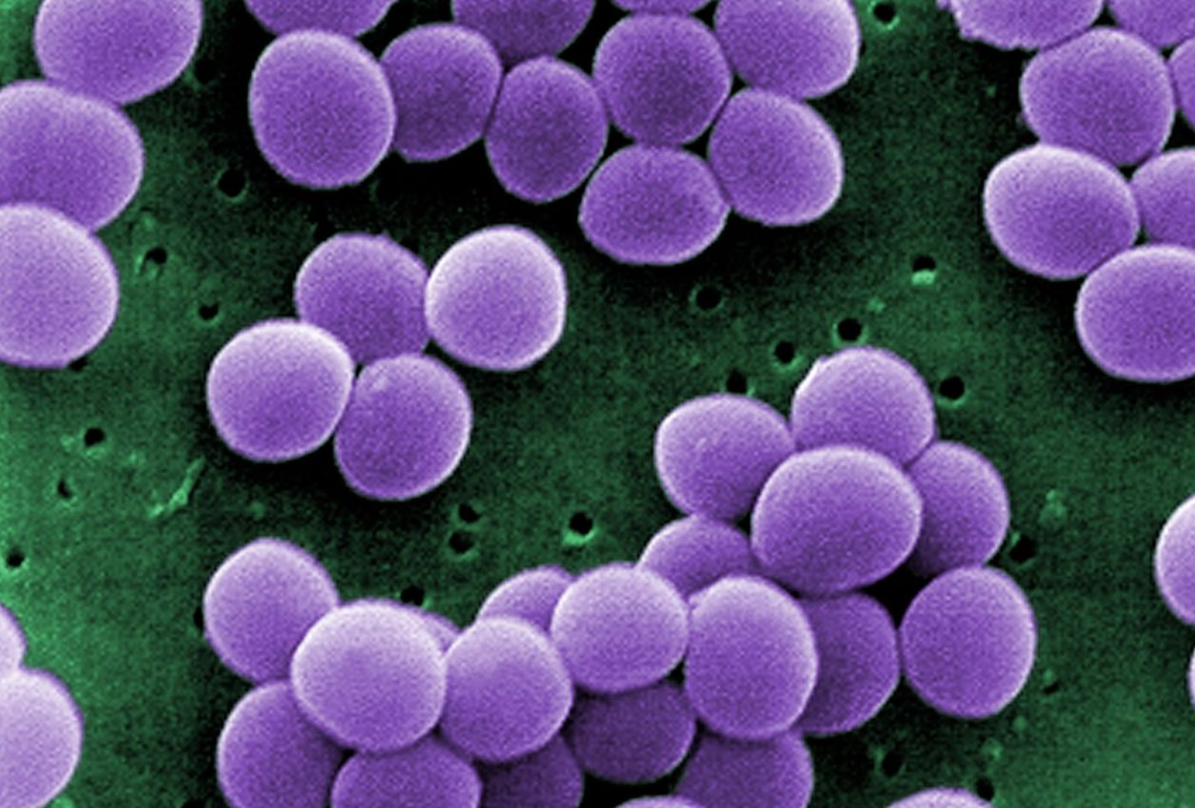
Scanning electron micrograph (SEM) showing a strain of Staphylococcus aureus bacteria taken from a vancomycin intermediate resistant culture (credit: CDC)
A team of researchers at MIT, the University of Brasilia, and the University of British Columbia has engineered an antimicrobial peptide to wipe out many types of bacteria, including some that are resistant to most antibiotics.
A recent study from a U.K. commission on antimicrobial resistance estimated that by 2050, antibiotic-resistant bacterial infections will kill 10 million people per year if no new drugs are developed.
Learning from nature
Living organisms naturally make antimicrobial peptides, as part of their immune system, to kill bacteria, as well as other persistent microbes such as viruses and fungi. It’s done by poking holes in the invaders’ cell membranes. Once inside, they can disrupt several cellular targets, including DNA, RNA, and proteins.
What also sets antimicrobial peptides apart from traditional antibiotics is their ability to recruit the host’s immune system, summoning cells called leukocytes that secrete chemicals that help kill the invading microbes.
In this new study, which appears in the Nov. 2 issue of Nature’s (open-access) Scientific Reports, the researchers began with a naturally occurring antimicrobial peptide called clavanin-A, which has proven deadly to many bacterial strains. The researchers decided to try to engineer it to make it even more effective.*
Killing resistant strains of E. coli and Staph
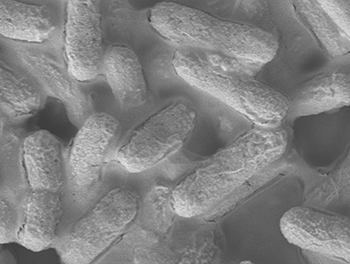
A microscopic image of E. coli bacteria (credit: A*STAR Institute of Bioengineering and Nanotechnology)
The researchers found in tests with mice that this new peptide, which they called clavanin-MO, could kill strains of Escherichia coli and Staphylococcus aureus that are resistant to most antibiotics.
The researchers also found that the clavanin-MO peptide suppresses the overactive inflammatory response that can cause sepsis, a life threatening condition, and can destroy certain biofilms (persistent thin layers of bacterial cells that form on surfaces). That raises the possibility of using them to treat infections caused by biofilms.**
The clavanin-MO peptide could also be embedded in surfaces such as tabletops to make them resistant to microbial growth, as antimicrobial coatings for catheters, and in ointments to treat skin infections caused by Staphylococcus aureus or other bacteria.
The researchers are now investigating what makes these engineered peptides more effective than the naturally occurring ones, with hopes of further improving them.
* Antimicrobial peptides have a positively charged region that allows them to poke through bacterial cell membranes, and a hydrophobic stretch that enables interaction with and translocation into membranes. The researchers decided to add a sequence of five amino acids that would make the peptides even more hydrophobic, in hopes that it would improve their killing ability.
** Such as the Pseudomonas aeruginosa infections that often affect the lungs of cystic fibrosis patients.
Abstract of An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities
Antibiotic-resistant infections are predicted to kill 10 million people per year by 2050, costing the global economy $100 trillion. Therefore, there is an urgent need to develop alternative technologies. We have engineered a synthetic peptide called clavanin-MO, derived from a marine tunicate antimicrobial peptide, which exhibits potent antimicrobial and immunomodulatory properties both in vitro and in vivo. The peptide effectively killed a panel of representative bacterial strains, including multidrug-resistant hospital isolates. Antimicrobial activity of the peptide was demonstrated in animal models, reducing bacterial counts by six orders of magnitude, and contributing to infection clearance. In addition, clavanin-MO was capable of modulating innate immunity by stimulating leukocyte recruitment to the site of infection, and production of immune mediators GM-CSF, IFN-γ and MCP-1, while suppressing an excessive and potentially harmful inflammatory response by increasing synthesis of anti-inflammatory cytokines such as IL-10 and repressing the levels of pro-inflammatory cytokines IL-12 and TNF-α. Finally, treatment with the peptide protected mice against otherwise lethal infections caused by both Gram-negative and -positive drug-resistant strains. The peptide presented here directly kills bacteria and further helps resolve infections through its immune modulatory properties. Peptide anti-infective therapeutics with combined antimicrobial and immunomodulatory properties represent a new approach to treat antibiotic-resistant infections.