New cancer-drug delivery system uses magnetically guided bacteria to target cancerous tumors with high precision
August 15, 2016
Artist’s impression of new cancer-drug nanotransporters using more than 100 million self-propelled, flagellated bacteria carrying nanoliposomes (green discs) loaded with drugs. The bacteria are guided by magnetic fields to take the most direct path between the drug’s injection point and the area of the body intended to cure. (credit: Montréal Nanorobotics Laboratory)
Researchers from Polytechnique Montréal, Université de Montréal, and McGill University have designed a new cancer-drug-delivery nanotransporter system using more than 100 million flagellated, self-propelled bacteria that are capable of navigating through the bloodstream to administer a drug to tumors with precision.* The goal of the research is to avoid jeopardizing the integrity of organs and surrounding healthy tissues while reducing drug dosage.
In an experiment with mice reported in the journal Nature Nanotechnology, the researchers confirmed that “the drug’s propelling force was enough to travel efficiently and enter deep inside the tumors,” autonomously detecting the oxygen-depleted tumor areas and delivering the drug to them, said Professor Sylvain Martel, holder of the Canada Research Chair in Medical Nanorobotics and Director of the Polytechnique Montréal Nanorobotics Laboratory, who heads the research team’s work.
Bacteria that detect both magnetic field lines and low-oxygen concentrations
In the experiment with mice, the researchers used liposomes as drug nanocarriers, attached to Magnetococcus marinus strain MC-1 bacteria to transport the drug**. In their natural environment, MC-1 bacteria tend to swim along local magnetic field lines, which are detected by a chain of magnetic iron-oxide nanocrystals in the bacteria. The bacteria also swim towards hypoxic (low-oxygen) concentrations. (In a tumor, this hypoxic zone is created by the substantial consumption of oxygen by rapidly proliferative tumor cells. Hypoxic zones are known to be resistant to most therapies, including radiotherapy.)
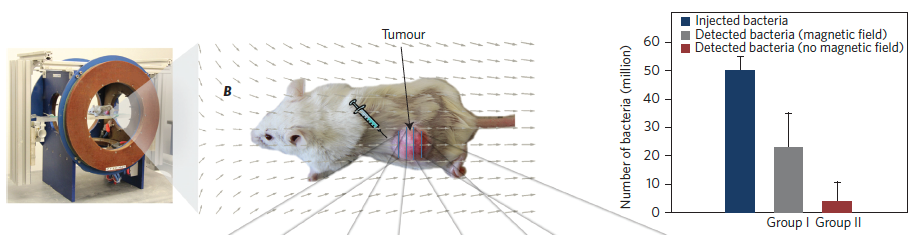
Test of penetration of live MC-1 bacteria cells with and without magnetic field exposure in xenografts following a peritumoral injection. (left) Magnetotaxis directional control system used to generate the magnetic field necessary to guide the MC-1 cells towards the xenograft. The directional magnetic field B was aligned towards the center of the tumoral volume. (center) MC-1 peritumoral injection into tumor xenograft in mice and representation of the applied directional magnetic field used in this study to direct the bacteria towards the xenograft. (right) Summary of the total average numbers of injected and detected MC-1 cells for all tumors in groups I and II. The results show that a significant number of the peritumorally injected MC-1 cells that were magnetically guided — using a relatively simple static directional magnetic field — were able to penetrate the xenograft, unlike the case for bacteria that were not guided into the tumor. (credit: Ouajdi Felfoul et al./Nature Nanotechnology)
The new nanotransporters used both of these natural systems (magnetic and hypoxic-concentration-seeking). The MC-1 bacteria first moved in the direction of a computer-controlled magnetic field to the point where oxygen gradients could be detected by the bacteria, enabling the bacteria to penetrate and remain in the tumor’s active regions. (In future human use, the drug could be released from the liposomes by sensing pH or particular enzymes, or by applying ultrasonics or heat, for example, the researchers explained to KurzweilAI.)
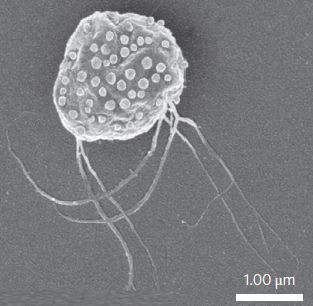
Scanning electron microscopy image of MC-1 bacterium with ~70 drug-bearing liposomes attached to the surface (credit: Ouajdi Felfoul et al./Nature Nanotechnology)
“This innovative use of nanotransporters will have an impact not only on creating more advanced engineering concepts and original intervention methods, but it also throws the door wide open to the synthesis of new vehicles for therapeutic, imaging, and diagnostic agents,” said Martel. Chemotherapy, which is so toxic for the entire human body, could make use of these natural nanotransporters to move drugs directly to the targeted area, “eliminating the harmful side effects while also boosting its therapeutic effectiveness,” he added.
The research was supported by the Consortium québécois sur la découverte du médicament (Québec consortium for drug discovery — CQDM), the Canada Research Chairs, the Natural Sciences and Engineering Research Council of Canada (NSERC), the Research Chair in Nanorobotics of Polytechnique Montréal, Mitacs, the Canada Foundation for Innovation (CFI), and the National Institutes of Health (NIH). Montréal’s Jewish General Hospital, the McGill University Health Centre (MUHC), the Institute for Research in Immunology and Cancer (IRIC), and the Rosalind and Morris Goodman Cancer Research Centre also participated.
* Only ∼2% of the total administered dose is deposited in the tumor with current delivery methods, according to a 2009 study by M. Hong et al. in J. Control. Rel., the researchers note in their Nature Nanotechnology paper.
** “Liposomes were selected as a first proof of concept because they are biocompatible, exhibit low immunogenicity and high flexibility, and protect the body from potential toxic cargo. Liposomes also shield therapeutic agents from premature degradation, control release kinetics, and may encapsulate a multitude of hydrophilic and∕or hydrophobic drug cargos, pharmaceutical ingredients, imaging agents and genetic material by virtue of their aqueous interior and lipid exterior,” the researchers note in their Nature Nanotechnology paper.
POLYTECHNIQUE MONTRÉAL | A Robotic Micro-assembly Process Inspired by the Construction of the Ancient Pyramids and Relying on Several Thousand of Flagellated Bacteria Acting as Workers
Abstract of Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions
Oxygen-depleted hypoxic regions in the tumour are generally resistant to therapies. Although nanocarriers have been used to deliver drugs, the targeting ratios have been very low. Here, we show that the magneto-aerotactic migration behaviour of magnetotactic bacteria, Magnetococcus marinus strain MC-1, can be used to transport drug-loaded nanoliposomes into hypoxic regions of the tumour. In their natural environment, MC-1 cells, each containing a chain of magnetic iron-oxide nanocrystals, tend to swim along local magnetic field lines and towards low oxygen concentrationsbased on a two-state aerotactic sensing system. We show that when MC-1 cells bearing covalently bound drug-containing nanoliposomes were injected near the tumour in severe combined immunodeficient beige mice and magnetically guided, up to 55% of MC-1 cells penetrated into hypoxic regions of HCT116 colorectal xenografts. Approximately 70 drug-loaded nanoliposomes were attached to each MC-1 cell. Our results suggest that harnessing swarms of microorganisms exhibiting magneto-aerotactic behaviour can significantly improve the therapeutic index of various nanocarriers in tumour hypoxic regions.