New live-cell printing technology improves on inkjet printing
February 11, 2014

Cells printed in a grid pattern by block cell printing technology (left) and woodblocks used in ancient Chinese printing (right) (credit: Lidong Qin lab and Digital Museum of Science and Art, Beijing, China)
A new way to print living cells onto any surface and in almost any shape has been developed by researchers led by Houston Methodist Research Institute nanomedicine faculty member Lidong Qin.
Unlike a similar inkjet printing process, almost all cells survive.
The new process, called Block-Cell-Printing (BloC-Printing), produces 2-D cell arrays in half an hour, prints the cells as close together as 5 microns (most animal cells are 10 to 30 microns wide), and allows the use of many different cell types.
“Cell printing is used in so many different ways now — for drug development and in studies of tissue regeneration, cell function, and cell-cell communication,” Qin said. “Such things can only be done when cells are alive and active. A survival rate of 50 to 80 percent is typical as cells exit the inkjet nozzles.
“By comparison, we are seeing close to 100 percent of cells in BloC-Printing survive the printing process.”
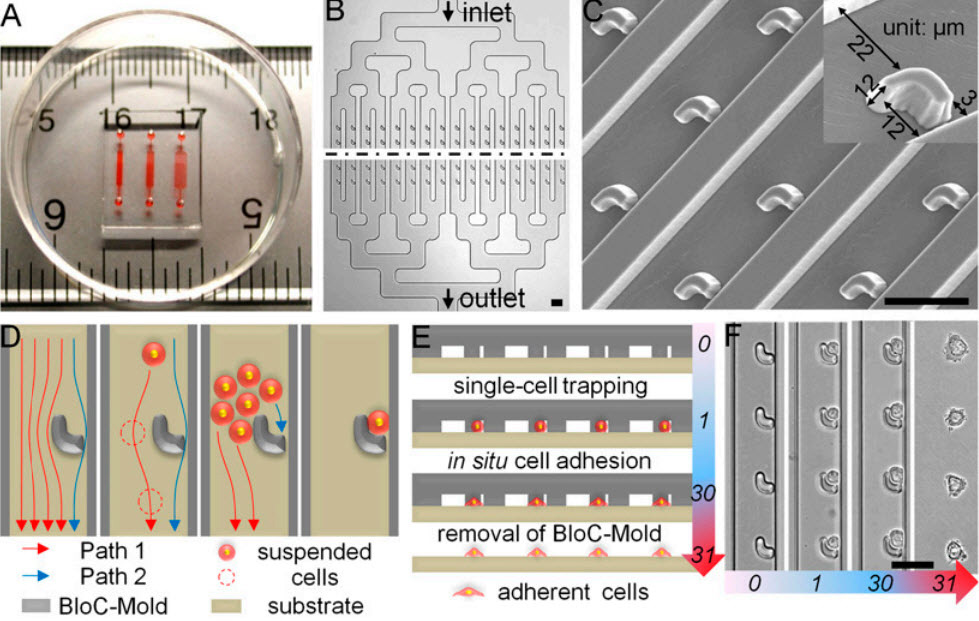
BloC-Printing manipulates microfluidic physics to guide living cells into hook-like traps in the silicone mold. Cells flow down a column in the mold, past trapped cells to the next available slot, eventually creating a line of cells in a grid.
The position and spacing of the traps and the shape of the channel navigated by the cells is fully configurable during the mold’s creation. When the mold is lifted away, the living cells remain behind, adhering to the growth medium or other substrate, in prescribed formation.
- Design and operation of BloC-Printing technique. (A) The device is displayed on a ruler to show scale, and red dye has been injected to aid visualization of the three distinct channel networks with trap spacings of 30, 50, and 90 microns, from left to right. (B) The BloC-Mold features symmetrical microfluidic channel networks and microarrays of traps. The black dashed line represents a large extended region between the input and output sides of the chip. (C) Scanning electron micrograph of the trap micro-array, taken at a 20° tilt-angle. A magnified, single trap is shown (Inset). (D) Schematic diagram of cell flow paths. Cross-sectional schematics (E) and corresponding bright-field micrographs (F) showing the entire BloC-Printing process: (i) single-cell trapping by the traps, (ii) in situ cell adhesion, and (iii) removal of the BloC-Mold. The numbers in E and F rep¬resent time in minutes. (Scale bars: 50 μm.) (Credit: Kai Zhanga et al./PNAS)
Qin’s group tested BloC-Printing for its utility in studying cancerous cells and primary neurons. By arranging metastatic cancer cells in a grid and examining their growth in comparison with a non-metastatic control, the researchers found they could easily characterize the metastatic potential of cancer cells.
“We looked at cancer cells for their protrusion generation capability, which correlates to their malignancy level,” Qin said. “Longer protrusion means more aggressive cancer cells. The measurement may help to diagnose a cancer’s stage.”
Printing neurons
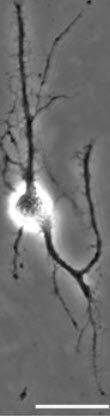
The researchers also printed a grid of brain cells and gave the cells time to form synaptic and autaptic junctions.
“The cell junctions we created may be useful for future neuron signal transduction and axon regeneration studies,” Qin said. “Such work could be helpful in understanding Alzheimer’s disease and other neurodegenerative diseases.”
While it is too early to predict the market cost of BloC-Printing, Qin said the materials of a single BloC mold cost about $1 (US). After the mold has been fabricated and delivered, a researcher only needs a syringe, a carefully prepared suspension of living cells, a Petri dish, and a steady hand, Qin said. Inkjet cell printers can cost between $10,000 and $200,000.
“BloC-Printing can be combined with molecular printing for many types of drug screening, RNA interference, and molecule-cell interaction studies,” he said. “We believe the technology has big potential.”
While the fidelity of BloC-Printing is high, Qin said inkjet printing remains faster, and BloC-Printing cannot yet print multi-layer structures as inkjetting can.
Qin and postdoctoral fellow Kai Zhang, Ph.D., are BloC-Printing’s co-inventors.
Support was provided by the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, the U.S. Dept. of Defense, the Emily Hermann Research Fund, the Golfers Against Cancer, and the Alliance for Nanohealth.
In addition to his position in the Houston Methodist Research Institute’s Department of Nanomedicine, Qin is also a Weill Cornell Medical College assistant professor of cell and developmental biology.
Abstract of Proceedings of the National Academy of Sciences paper
A unique live-cell printing technique, termed “Block-Cell-Printing” (BloC-Printing), allows for convenient, precise, multiplexed, and high-throughput printing of functional single-cell arrays. Adapted from woodblock printing techniques, the approach employs microfluidic arrays of hook-shaped traps to hold cells at designated positions and directly transfer the anchored cells onto various substrates. BloC-Printing has a minimum turnaround time of 0.5 h, a maximum resolution of 5 µm, close to 100% cell viability, the ability to handle multiple cell types, and efficiently construct protrusion-connected single-cell arrays. The approach enables the large-scale formation of heterotypic cell pairs with controlled morphology and allows for material transport through gap junction intercellular communication. When six types of breast cancer cells are allowed to extend membrane protrusions in the BloC-Printing device for 3 h, multiple biophysical characteristics of cells—including the protrusion percentage, extension rate, and cell length—are easily quantified and found to correlate well with their migration levels. In light of this discovery, BloC-Printing may serve as a rapid and high-throughput cell protrusion characterization tool to measure the invasion and migration capability of cancer cells. Furthermore, primary neurons are also compatible with BloC-Printing.