A new material for creating artificial blood vessels
April 29, 2015
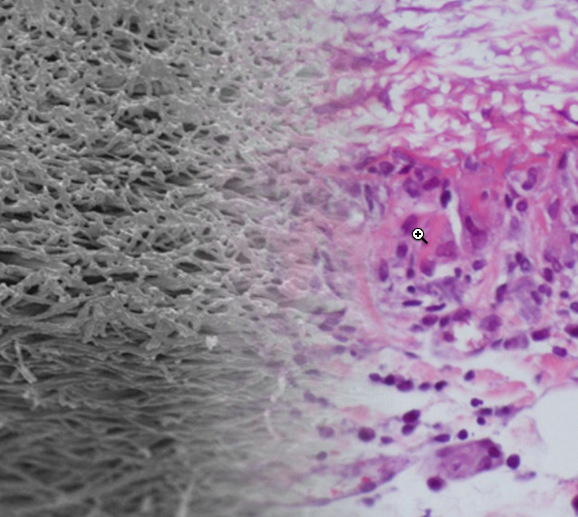
The artificial blood-vessel material (left) combines well with the natural biomaterial (right) in this photo montage (credit: TU Wien)
Vienna University of Technology (TU Wien) and Vienna Medical University (MedUni Vienna) researchers have developed artificial blood vessels made from a special elastomer material (thermoplastic polyurethanes) with excellent mechanical properties.
The artificial blood vessels are designed to be broken down by the body and replaced with its own tissue. At the end of this restorative process, a natural, fully functional vessel will be once again in place.
Arteriosclerotic vascular disorder is one of the most common causes of death in industrialized countries. A bypass operation is often the only solution. Normally, blood vessels are taken from another part of the patient’s body and used to replace the damaged vessel.
The artificial materials used so far are not fully compatible with body tissue, and the blood vessel can easily become blocked.
Rat experiments successful
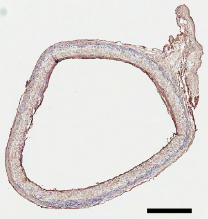
Cross-section of implanted artificial blood vessel graft (at 12 months) without signs of aneurysm or intimal hyperplasia (scale bar: 500 micrometers).
To produce the new vascular prostheses, polymer solutions were spun in an electrical field to form very fine threads and wound onto a spool. “The wall of these artificial blood vessels is very similar to that of natural ones,” says Heinz Schima of the Medical University of Vienna.
The polymer fabric is slightly porous, so it allows a small amount of blood to permeate the material. This enriches the wall with growth factors, encouraging migration of endogenous (body’s own) cells.
The new method has proved successful in experiments with rats. “The rats’ blood vessels were examined six months after insertion of the vascular prostheses,” says Helga Bergmeister of MedUni Vienna.
“We did not find any aneurysms, thromboses, or inflammation. Endogenous cells had colonized the vascular prostheses and turned the artificial constructs into natural body tissue.”
A few more preclinical trials are necessary before the artificial blood vessels can be used in humans, which the researchers expect in a few years.
Abstract of Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements
Biodegradable vascular grafts with sufficient in vivo performance would be more advantageous than permanent non-degradable prostheses. These constructs would be continuously replaced by host tissue, leading to an endogenous functional implant which would adapt to the need of the patient and exhibit only limited risk of microbiological graft contamination. Adequate biomechanical strength and a wall structure which promotes rapid host remodeling are prerequisites for biodegradable approaches. Current approaches often reveal limited tensile strength and therefore require thicker or reinforced graft walls. In this study we investigated the in vitro and in vivo biocompatibility of thin host-vessel-matched grafts (n = 34) formed from hard-block biodegradable thermoplastic polyurethane (TPU). Expanded polytetrafluoroethylene (ePTFE) conduits (n = 34) served as control grafts. Grafts were analyzed by various techniques after retrieval at different time points (1 week; 1, 6, 12 months). TPU grafts showed significantly increased endothelial cell proliferation in vitro (P < 0.001). Population by host cells increased significantly in the TPU conduits within 1 month of implantation (P = 0.01). After long-term implantation, TPU implants showed 100% patency (ePTFE: 93%) with no signs of aneurysmal dilatation. Substantial remodeling of the degradable grafts was observed but varied between subjects. Intimal hyperplasia was limited to ePTFE conduits (29%). Thin-walled TPU grafts offer a new and desirable form of biodegradable vascular implant. Degradable grafts showed equivalent long-term performance characteristics compared to the clinically used, non-degradable material with improvements in intimal hyperplasia and ingrowth of host cells.