New material kills E. coli bacteria in 30 seconds
June 6, 2016
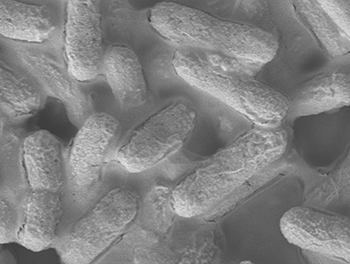
A microscopic image of the E. coli bacteria (credit: Institute of Bioengineering and Nanotechnology)
A new material that can kill E. coli bacteria within 30 seconds has been developed by researchers at the Institute of Bioengineering and Nanotechnology (IBN) of A*STAR in Singapore.
Triclosan, a common antibacterial ingredient found in many products such as toothpastes, soaps, and detergents to reduce or prevent bacterial infections, has been linked to making bacteria resistant to antibiotics, with adverse health effects. The European Union has restricted the use of triclosan in cosmetics, and the U.S. FDA is conducting an ongoing review of this ingredient.
Destroying the cell membrane to prevent resistance to antibiotics
To find a more suitable alternative, IBN Group Leader Yugen Zhang, PhD, and his team synthesized a chemical compound made up of molecules linked together in a chain (“imidazolium oligomers”), which they found can kill 99.7% of the E. coli bacteria within 30 seconds. The chain-like structure helps to penetrate the cell membrane and destroy the bacteria.
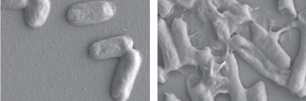
SEM image of E. coli before (left) and after treatment with IBN-C8 (right) (credit: S.N. Riduan et al./Small)
In contrast, current antibiotics only kill the bacteria but fail to destroy the cell membrane, allowing new antibiotic-resistant bacteria to grow.
“Our unique material can kill bacteria rapidly and inhibit the development of antibiotic-resistant bacteria. Computational chemistry studies supported our experimental findings that the chain-like compound works by attacking the cell membrane. This material is also safe for use because it carries a positive charge that targets the more negatively charged bacteria, without destroying red blood cells,” said Zhang.
The imidazolium oligomers come in the form of a white powder that is soluble in water. The researchers also found that once this was dissolved in alcohol, it formed gels spontaneously. So this material could be incorporated in alcohol-based sprays used for sterilization in hospitals or homes.
E. coli is a type of bacteria found in the intestines of humans and animals, and some strains can cause severe diarrhea, abdominal pain, and fever. Such infection is contagious and can spread through contaminated food or water, or from contact with people or animals.
Besides E. coli, IBN’s material was tested against other common strains of antibiotic-resistant bacteria and fungi such as Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans (which can cause conditions ranging from skin infections to pneumonia and toxic shock syndrome) and was able to kill 99.9% of these microbes within two minutes.
The finding was published in the peer-reviewed journal Small.
Abstract of Ultrafast Killing and Self-Gelling Antimicrobial Imidazolium Oligomers
Infectious diseases and the increasing threat of worldwide pandemics have underscored the importance of antibiotics and hygiene. Intensive efforts have been devoted to developing new antibiotics to meet the rapidly growing demand. In particular, advancing the knowledge of the structure–property–activity relationship is critical to expedite the design and development of novel antimicrobial with the needed potential and efficacy. Herein, a series of new antimicrobial imidazolium oligomers are developed with the rational manipulation of terminal group’s hydrophobicity. These materials exhibit superior activity, excellent selectivity, ultrafast killing (>99.7% killing within 30 s), and desirable self-gelling properties. Molecular dynamic simulations reveal the delicate effect of structural changes on the translocation motion across the microbial cell membrane. The energy barrier of the translocation process analyzed by free energy calculations provides clear kinetic information to suggest that the spontaneous penetration requires a very short timescale of seconds to minutes for the new imidazolium oligomers.