New microscope creates 3D movies of living things
January 21, 2015
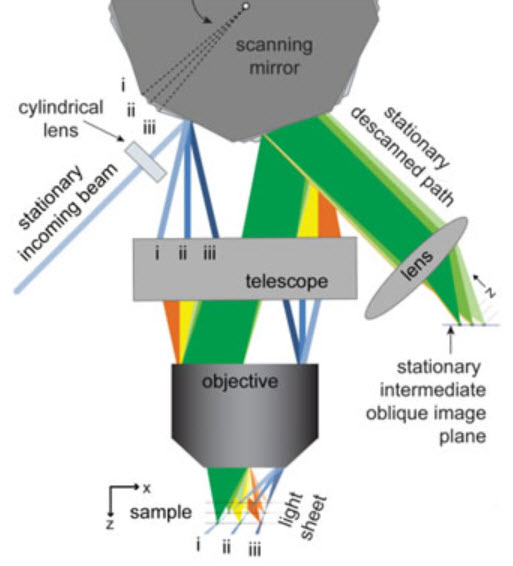
This schematic depicts SCAPE’s imaging geometry. The light sheet is swept at the sample by slowly moving a polygonal mirror mounted on a galvanometer motor. This alters the angle at which the light is incident at the edge of the objective’s back aperture, causing the beam to sweep across the sample. The light emitted by fluorophores within this illuminated plane travels back through the same objective lens, and is de-scanned by the same polygonal mirror (from an adjacent facet). This light forms an oblique image of the illuminated plane that stays stationary and aligned with the illumination plane, even though the light sheet is moving through the sample (just as a confocal pinhole stays aligned with the scanning illuminated focal point in laser scanning confocal microscopy). So with one (<5 degree) movement of the polygon, the entire volume is sampled. (Credit: Elizabeth Hillman, Columbia Engineering)
A Columbia University scientist has developed a new microscope that can image freely moving living things in 3D at very high speeds — up to 100 times faster 3D imaging than laser-scanning confocal, two-photon, and light-sheet microscopy.
Developed by Elizabeth Hillman, associate professor of biomedical engineering at Columbia Engineering, SCAPE (swept confocally aligned planar excitation microscopy) uses a simple, single-objective lens imaging geometry that requires no sample mounting or translation (movement).
The issue Hillman and other researchers are dealing with here is that while current confocal and two-photon microscopes do a good job of imaging a single plane within a living sample, it’s difficult to acquire enough of these layers to form a 3D image at fast enough rates to capture events like neurons actually firing.
“With SCAPE, we can now image complex, living things, such as neurons firing in the rodent brain, crawling fruit fly larvae, and single cells in the zebrafish heart while the heart is actually beating spontaneously — this has not been possible until now,” she says.
SCAPE is actually a variation on light-sheet imaging, but it “breaks all the rules,” says Hillman. While conventional light-sheet microscopes use two awkwardly positioned objective lenses, Hillman realized that she could use a single-objective lens, and then that she could sweep the light sheet to generate 3D images without moving the objective or the sample.
SCAPE can’t yet compete with the penetration depth of conventional two-photon microscopy, but Hillman and her collaborators have already used the system to observe firing in 3D neuronal dendritic trees in superficial layers of the mouse brain, formerly impossible.
In small organisms, including zebrafish larvae, SCAPE can see through the entire organism. By tracking these tiny, unrestrained creatures in 3D at high speeds, SCAPE can capture both cellular structure and function and behavior, because it doesn’t require time-consuming movement of the imaging objective lens or the sample to create a 3D image. SCAPE can also be combined with optogenetics and other tissue manipulations during imaging.
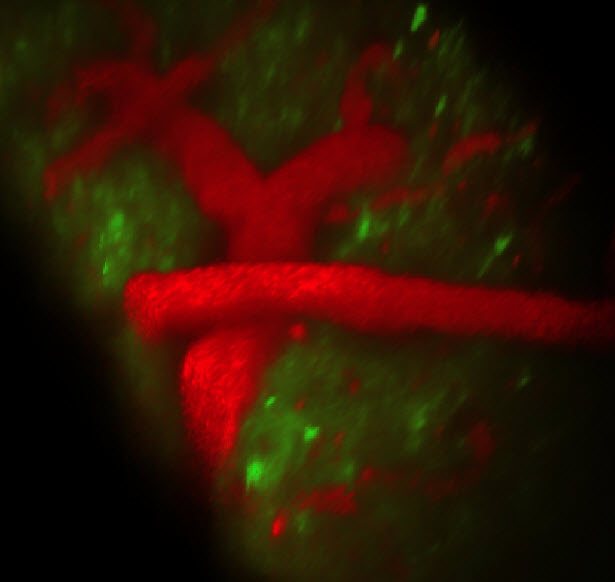
SCAPE imaging of the living brain, with green GCaMP labeling apical dendrites of layer 5 neurons, and red showing Texas red dextran in the vasculature. Data was acquired at 10 volumes per second. (Credit: Elizabeth Hillman and Clay Lacefield)
Her study was published in the Advance Online Publication (AOP) on Nature Photonics’s website on Monday, January 19.
How to make a high-speed microscope
Hillman and her students built their first SCAPE system using inexpensive off-the-shelf components. Her “aha” moment came when, looking at an old polygonal mirror in the lab, she realized how it could be used to generate SCAPE’s unusual scanning geometry.
After several years of trial and error, Hillman and graduate student Matthew Bouchard came up with a configuration that worked, and beautiful images started to flow out. “It wasn’t until we built it that we realized it was a light-sheet microscope!” says Hillman.
“It took us a while to realize how versatile the imaging geometry was, how simple and inexpensive the layout was—and just how many problems we had overcome.”
Extensive applications in biology and medicine
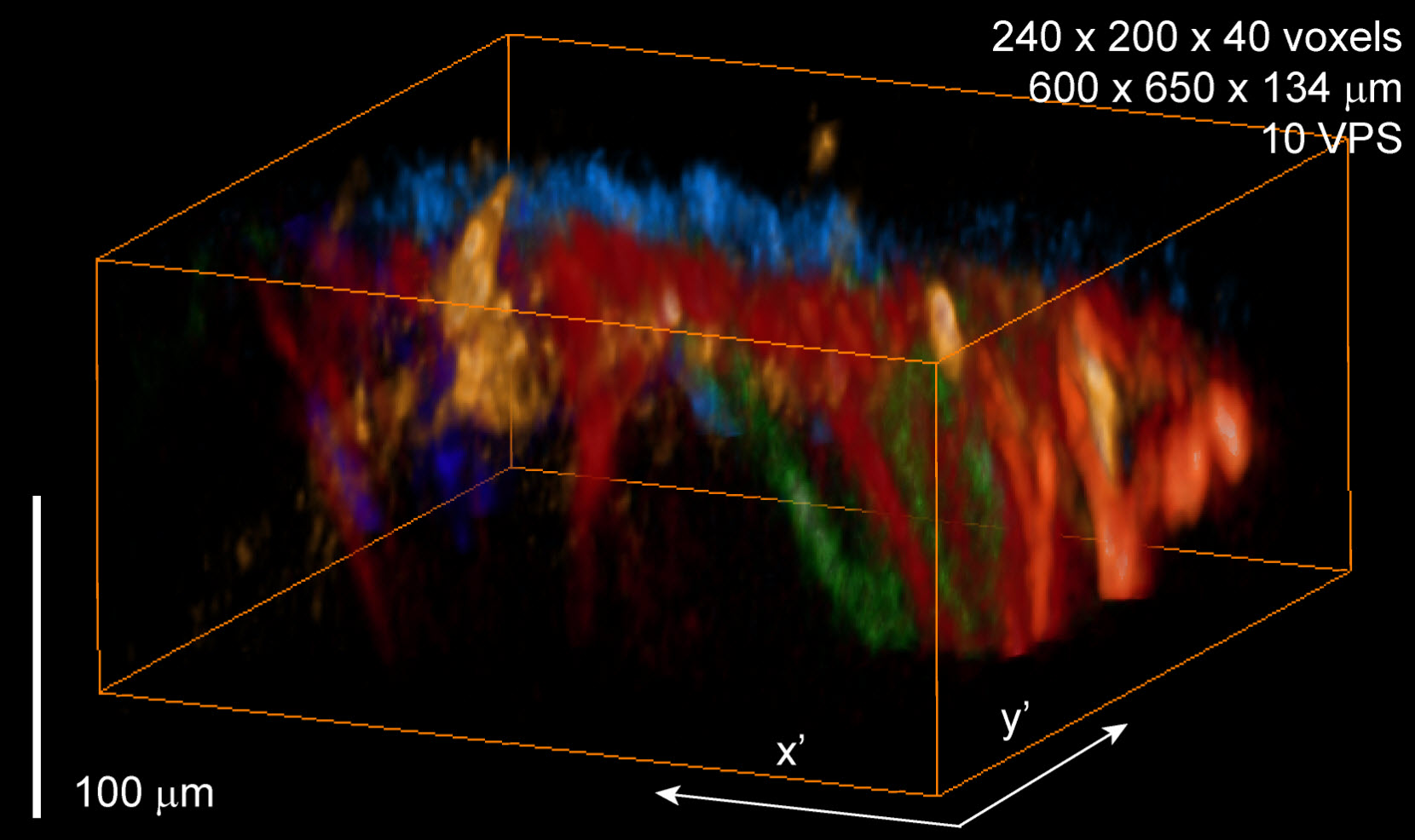
Renderings of 8 different color-coded dendrites observed to flash during spontaneous events (credit: Elizabeth Hillman, Matthew Bouchard, Venkatakaushik Voleti, Clay Lacefield and Randy Bruno)
Beyond neuroscience, Hillman sees many future applications of SCAPE including imaging cellular replication, function, and motion in intact tissues, 3D cell cultures, and engineered tissue constructs, as well as imaging 3D dynamics in microfluidics, and flow-cell cytometry systems. These are all applications where molecular biology is delivering tools and techniques, but imaging methods have struggled to keep up.
Hillman also plans to explore clinical applications of SCAPE such as video-rate 3D microendoscopy and intrasurgical imaging.
Next-generation versions of SCAPE are in development that will deliver even better speed, resolution, sensitivity, and penetration depth. Hillman’s technology is available for licensing from Columbia Technology Ventures and has already attracted interest from multiple companies.
This research was supported by grants from NIH (NINDS), the Human Frontier Science Program, the Wallace H. Coulter Foundation (E.M.C.H), the Dana Foundation (R.M.B.), and DoD.
A patent related to this technique was issued on December 31, 2013 (inventors Hillman and Bouchard). The authors are currently in licensing discussions.
Hillman is also associate professor of radiology at Columbia University Medical Center, associate professor of radiology at Columbia University Medical Center, and a member of Columbia’s Mortimer B. Zuckerman Mind Brain Behavior Institute.
Hillman Lab, Columbia University | SCAPE real-time 3D microscopy — Intact brain
Hillman Lab, Columbia University | SCAPE real-time 3D microscopy — Beating zebrafish heart
Abstract of Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms
We report a three-dimensional microscopy technique—swept, confocally-aligned planar excitation (SCAPE) microscopy—that allows volumetric imaging of living samples at ultrahigh speeds. Although confocal and two-photon microscopy have revolutionized biomedical research, current implementations are costly, complex and limited in their ability to image three-dimensional volumes at high speeds. Light-sheet microscopy techniques using two-objective, orthogonal illumination and detection require a highly constrained sample geometry and either physical sample translation or complex synchronization of illumination and detection planes. In contrast, SCAPE microscopy acquires images using an angled, swept light sheet in a single-objective, en face geometry. Unique confocal descanning and image rotation optics map this moving plane onto a stationary high-speed camera, permitting completely translationless three-dimensional imaging of intact samples at rates exceeding 20 volumes per second. We demonstrate SCAPE microscopy by imaging spontaneous neuronal firing in the intact brain of awake behaving mice, as well as freely moving transgenic Drosophila larvae.