New self-healing polymers require no chemicals or catalysts
February 12, 2014
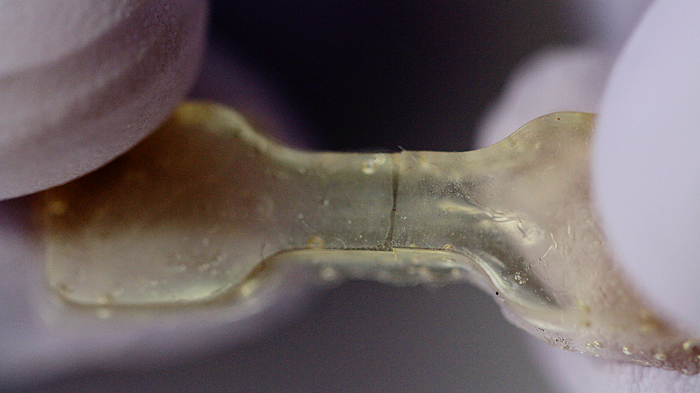
This elastic polymer was cut in two and healed overnight. (credit: Anne Lukeman)
University of Illinois researchers have developed new self-healing materials that do not require extra chemicals or catalysts.
“The key advantage of using this material is that it’s catalyst-free and low-temperature, and can be healed multiple times,” said U. of I. materials science and engineering professor Jianjun Cheng. “This can heal the crack before it causes major problems by propagating.”
Other self-healing material systems have focused on solid, strong materials. The new study uses softer elastic materials made of polyurea, one of the most widely used classes of polymers in consumer goods such as paints, coatings, elastics and plastics.
‘Dynamic chemistry’
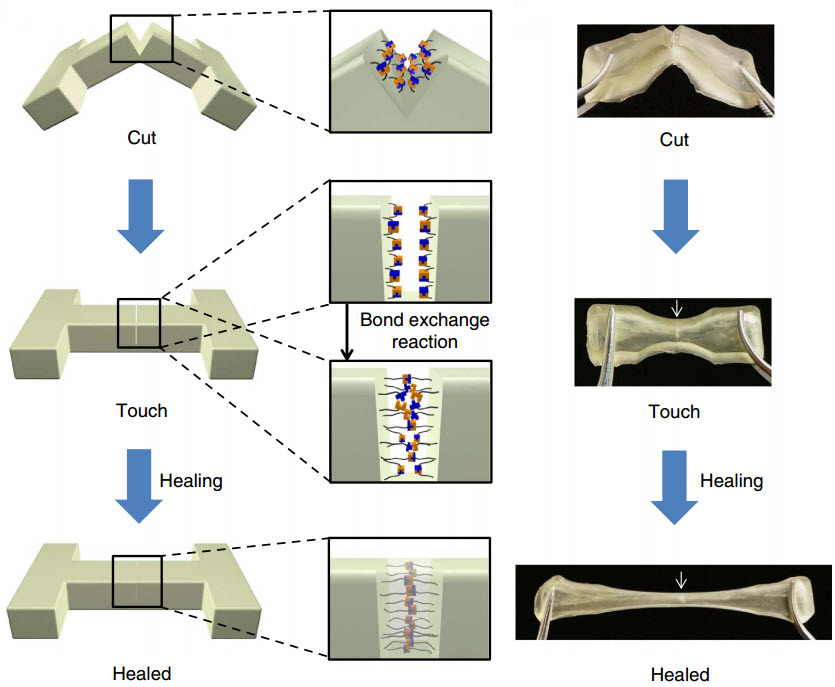
If the polymer is cut or torn, the researchers can simply press the two pieces back together and let the sample sit for about a day to heal — no extra chemicals or catalysts required. The materials can heal at room temperature, but the process can be sped up by curing at slightly higher temperatures (37 degrees Celsius, or about body temperature).
The “hindered urea bond” polymer bonds back together on the molecular level nearly as strongly as before it was cut. In fact, tests found that some healed samples, stretched to their limits, tore in a new place rather than the healed spot, evidence that the samples had healed completely.
The researchers use commercially available ingredients to create their polymer. By slightly tweaking the structure of the molecules that join up to make the polymer, they can make the bonds between the molecules longer so that they can more easily pull apart and stick back together — the key for healing. This molecular-level re-bonding is called “dynamic chemistry.”
Commercial materials
“We just buy commercial materials and mix them together, no fancy controls or special apparatus,” said Cheng. “It’s a very simple, low-cost, inexpensive process. Anybody can do this on any scale.”
Now that they’ve established the chemistry required, the researchers are exploring how dynamic polyurea could bolster different applications. For example, they could fine-tune the mixture so that a polyurethane coating or paint could be removable.
“In some areas, when it’s not necessary for the coating to be permanent and you want it to be removable, this chemistry may be applied to existing coating materials to make it reversible,” Cheng said. “In general, polyurea and polyurethane are widely used. This chemistry could modify existing materials to make them more dynamic, healable.”
The National Science Foundation and the National Institutes of Health supported this research.
Abstract of Nature Communications paper
Polymers bearing dynamic covalent bonds may exhibit dynamic properties, such as self-healing, shape memory and environmental adaptation. However, most dynamic covalent chemistries developed so far require either catalyst or change of environmental conditions to facilitate bond reversion and dynamic property change in bulk materials. Here we report the rational design of hindered urea bonds (urea with bulky substituent attached to its nitrogen) and the use of them to make polyureas and poly(urethane-urea)s capable of catalyst-free dynamic property change and autonomous repairing at low temperature. Given the simplicity of the hindered urea bond chemistry (reaction of a bulky amine with an isocyanate), incorporation of the catalyst-free dynamic covalent urea bonds to conventional polyurea or urea-containing polymers that typically have stable bulk properties may further broaden the scope of applications of these widely used materials.