New stem-cell-derived cells hold promise for Alzheimer’s, other brain diseases
November 9, 2012
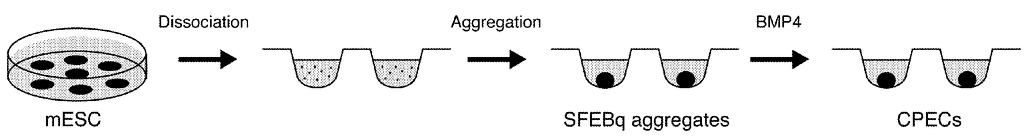
Choroid plexus epithelial cells generated in a culture medium using embryonic stem cells
(credit: Edwin S. Monuki and Momoko Watanabe/USPTO)
UC Irvine researchers have created a new stem cell-derived cell type with unique promise for treating neurodegenerative diseases such as Alzheimer’s.
Dr. Edwin Monuki of UCI’s Sue & Bill Gross Stem Cell Research Center and colleagues developed these cells — called choroid plexus epithelial cells (CPECs) — from existing mouse and human embryonic stem cell lines.
CPECs are critical for proper functioning of the choroid plexus, the tissue in the brain that produces cerebrospinal fluid (CSF ). CPECs make CSF and remove metabolic waste and foreign substances from the fluid and brain, among other tasks.
In neurodegenerative diseases, the choroid plexus and CPECs age prematurely, resulting in reduced CSF formation and decreased ability to flush out the plaque-forming proteins that are a hallmark of Alzheimer’s. Transplant studies have provided proof of concept for CPEC-based therapies. However, such therapies have been hindered by the inability to expand or generate CPECs in culture.
“Our method is promising, because for the first time we can use stem cells to create large amounts of these epithelial cells, which could be utilized in different ways to treat neurodegenerative diseases,” said Monuki, an associate professor of pathology & laboratory medicine and developmental & cell biology at UCI.
To create the new cells, Monuki and his colleagues coaxed embryonic stem cells to differentiate into immature neural stem cells. They then developed the immature cells into CPECs capable of being delivered to a patient’s choroid plexus.
These cells could be part of neurodegenerative disease treatments in at least three ways, Monuki said. First, they’re able to increase the production of CSF to help flush out plaque-causing proteins from brain tissue and limit disease progression. Second, CPEC “superpumps” could be designed to transport high levels of therapeutic compounds to the CSF, brain and spinal cord. Third, these cells can be used to screen and optimize drugs that improve choroid plexus function.
Monuki said the next steps are to develop an effective drug screening system and to conduct proof-of-concept studies to see how these CPECs affect the brain in mouse models of Huntington’s, Alzheimer’s and pediatric diseases.
The study as supported by the National Institutes of Health, the California Institute for Regenerative Medicine, UCI’s Institute for Clinical & Translational Science, and UCI’s Alzheimer’s Disease Research Center.