New way to target — and kill — proliferating tumors
November 16, 2011
Researchers at the University of California, San Diego School of Medicine and the UC San Diego Moores Cancer Center have identified a new drug-discovery approach enabling the destruction of the most highly proliferative tumors, pointing to an effective, alternative method for killing fast-growing cancer cells without causing some of the negative effects of current therapies.
The scientists, led by David A. Cheresh, PhD, professor of pathology and associate director for translational research at the Moores Cancer Center, used an innovative chemical and biological approach to design a new class of drugs that arrests division in virtually all tumor cells by binding to and altering the structure of an enzyme called RAF.
RAF has been long-studied, but its role in cell division — critical to cell proliferation and tumor growth — was a surprise. “By designing a new class of drugs that changes the shape of RAF, we were able to reveal this previously undiscovered role for RAF in a wide range of highly proliferative tumors,” Cheresh said.
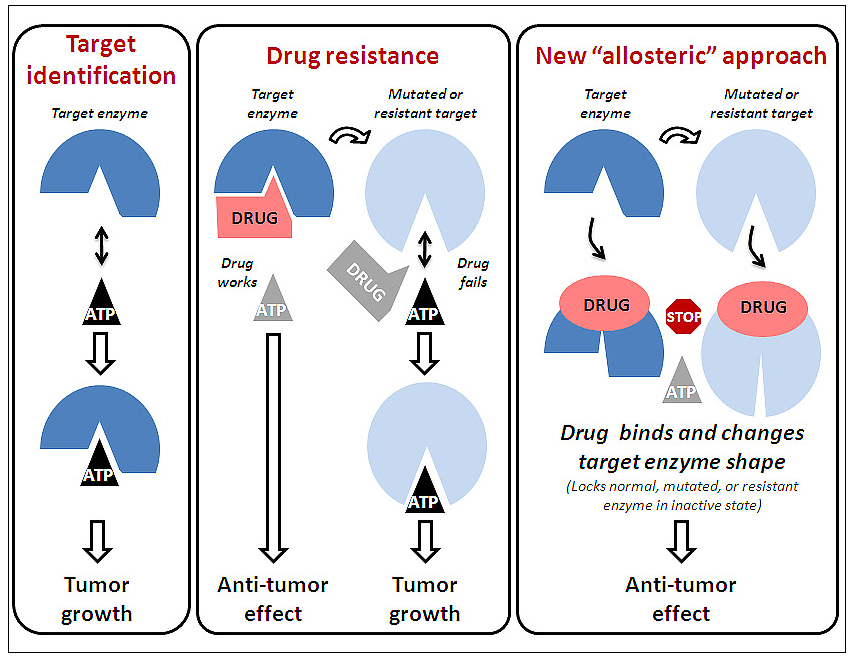
Current cancer drugs that target enzymes like RAF are generally designed to interact with the active site of the enzyme. Unfortunately, these drugs often lack specificity, Cheresh said. “They hit many different targets, meaning they can produce undesired side effects and induce dose-limiting toxicity.” More of a concern is that tumor cells often develop resistance to this class of drugs rendering them inactive against the cancer.
Cheresh and colleagues pursued development of a new class of RAF inhibitors that do not bind to the active site of the enzyme and so avoid the limitations of current drugs. Instead, this new class, called allosteric inhibitors, changes the shape of the target enzyme and in doing so, renders it inactive. The specific drug tested, known as KG5, singles out RAF in proliferating cells, but ignores normal or resting cells. In affected tumor cells, RAF is unable to associate with the mitotic apparatus to direct cell division, resulting in cell cycle arrest leading to apoptosis or programmed cell death. KG5 in a similar manner effectively interferes with proliferating blood vessels, a process called angiogenesis.
“It’s an unusual discovery, one that really challenges current dogma,” said Cheresh. “Before this drug was designed, we had no idea RAF could promote tumor cell cycle progression. This may be only one example of how, by designing drugs that avoid the active site of an enzyme, we can identify new and unexpected ways to disrupt the growth of tumors. In essence, we are attacking an important enzyme in a whole new way and thereby discovering new things this enzyme was intended for.”
KG5 produced similar results in tests on cancer cell lines, in animal models and in tissue biopsies from human cancer patients. The research team has since developed variants of KG5 that are 100-fold more powerful than the original drug. They hope one of these more powerful compounds will soon enter clinical trials at Moores Cancer Center.
Ref.: Ainhoa Mielgo, et al., A MEK-independent role for CRAF in mitosis and tumor progression, Nature Medicine, 2011; [DOI: 10.1038/nm.2464]