Optogenetics captures synaptic transmission in live mammalian brain for the first time
December 26, 2014
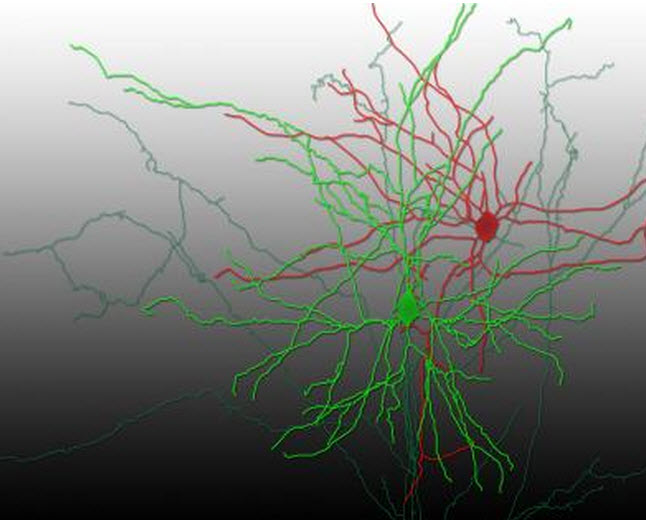
A reconstruction of a pair of synaptically connected neurons (credit: Aurélie Pala/EPFL)
EPFL scientists Aurélie Pala and Carl Petersen have observed and measured synaptic transmission in a live animal for the first time, using optogenetics* to stimulate single neurons in the mouse barrel cortex (which processes sensory information from the mouse’s whiskers).
They shined blue light on the neurons containing a gene-based light-sensitive protein, activating the neurons to fire. Then using microelectrodes, they measured resulting electrical signals in neighboring interneuron cells.
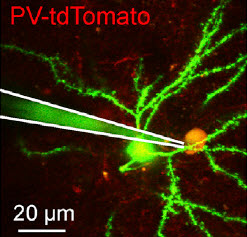
Excitatory synaptic transmission in vivo: Whole-cell recording of excitatory postsynaptic potentials (voltages) elicited in an excitatory presynaptic neuron during neuronal network quiescence (inactive phase) by light pulses. The in vivo two-photon image shows the whole-cell recording pipette (white lines) connected to a “PV-tdTomato” gene-expressing neuron (yellow) being recorded and part of the presynaptic gene-expressing neuron (green) (credit: Aurélie Pala/EPFL)
They also used an advanced imaging technique (two-photon microscopy) that allowed them to look deep into the brain of the live mouse and identify the type of each interneuron they were studying.
The data showed that the neuronal transmissions from the light-sensitive neurons differed depending on the type of interneuron on the receiving end.
Only a few studies have directly investigated synaptic transmission between specific neocortical neurons in vivo, presumably due to the technical difficulties in obtaining intracellular recordings from connected pairs of neurons in vivo, the authors say in their (open-access) paper in Neuron.
The research overcomes a limitation of in vitro (lab) studies, where associated biomolecules are different from those in a live animal, and where cutting neural tissue for lab work also introduces artifacts, the researchers suggest.
“This is a proof-of-concept study,” says Pala, who received her PhD for this work. “Nonetheless, we think that we can use optogenetics to put together a larger picture of connectivity between other types of neurons in other areas of the brain.”
The scientists are now aiming to explore other neuronal connections in the mouse barrel cortex. They also want to try this technique on awake mice, to see how switching neuronal activity on and off with a light can affect higher brain functions.
* Optogenetics works by inserting the gene of an light-sensitive protein into live neurons, from a single cell to an entire family of them. The genetically modified neurons then produce the light-sensitive protein, which sits on their outside, the membrane. There, it acts as an electrical channel – something like a gate. When light is shone on the neuron, the channel opens up and allows electrical ions to flow into the cell; a bit like a battery being charged by a solar cell.
The addition of electrical ions changes the voltage balance of the neuron, and if the optogenetic stimulus is sufficiently strong it generates an explosive electrical signal in the neuron.
Abstract of In Vivo Measurement of Cell-Type-Specific Synaptic Connectivity and Synaptic Transmission in Layer 2/3 Mouse Barrel Cortex
Intracellular recordings of membrane potential in vitro have defined fundamental properties of synaptic communication. Much less is known about the properties of synaptic connectivity and synaptic transmission in vivo. Here, we combined single-cell optogenetics with whole-cell recordings to investigate glutamatergic synaptic transmission in vivo from single identified excitatory neurons onto two genetically defined subtypes of inhibitory GABAergic neurons in layer 2/3 mouse barrel cortex. We found that parvalbumin-expressing (PV) GABAergic neurons received unitary glutamatergic synaptic input with higher probability than somatostatin-expressing (Sst) GABAergic neurons. Unitary excitatory postsynaptic potentials onto PV neurons were also faster and more reliable than inputs onto Sst neurons. Excitatory synapses targeting Sst neurons displayed strong short-term facilitation, while those targeting PV neurons showed little short-term dynamics. Our results largely agree with in vitro measurements. We therefore demonstrate the technical feasibility of assessing functional cell-type-specific synaptic connectivity in vivo, allowing future investigations into context-dependent modulation of synaptic transmission.