Parkinson’s disease researchers discover a way to reprogram the genome to produce dopamine neurons
December 8, 2015
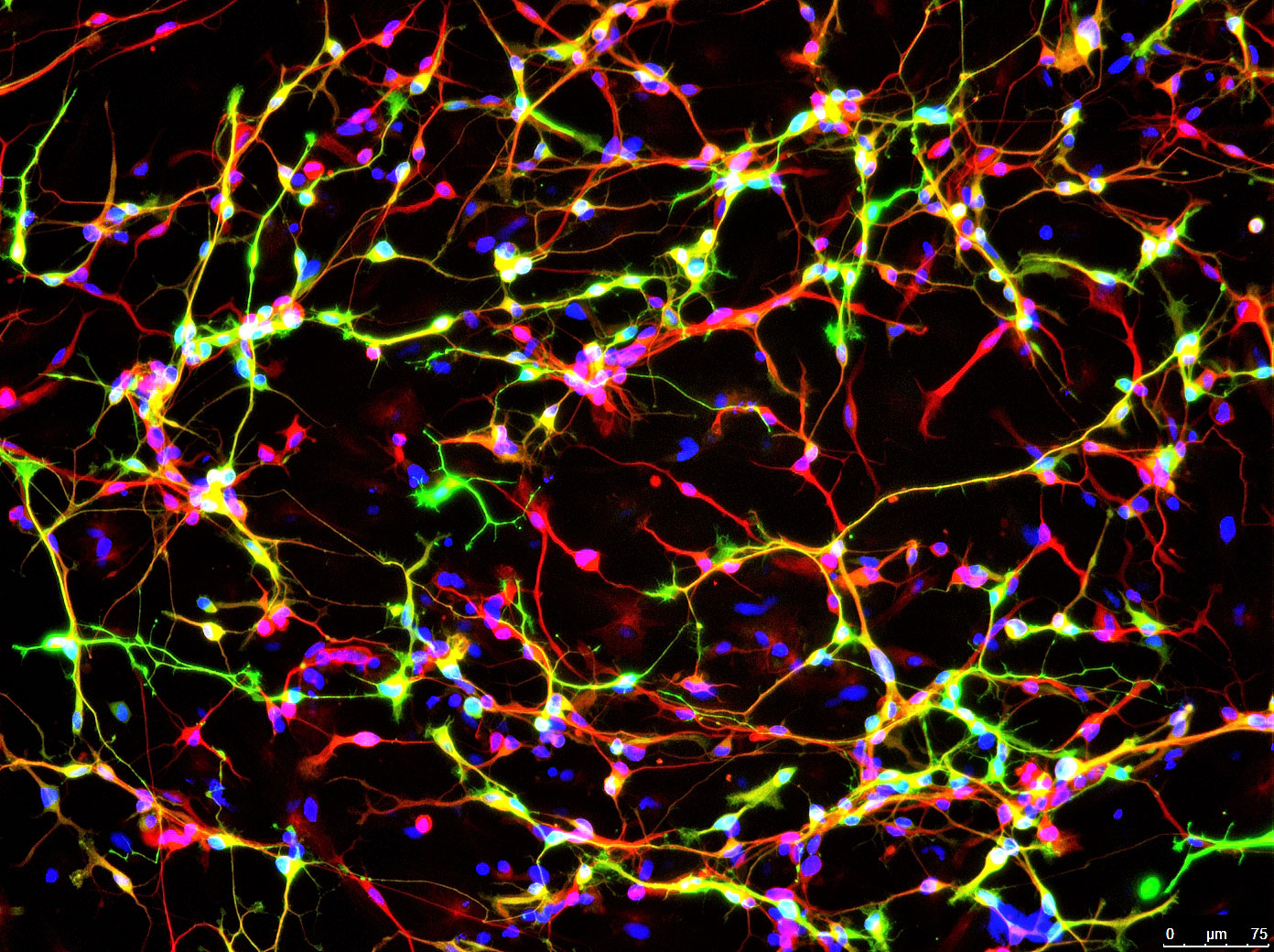
Image shows a protein found only in neurons (red) and an enzyme that synthesizes dopamine (green). Cell DNA is labeled in blue. (credit: Jian Feng, University at Buffalo)
Parkinson’s disease researchers at the Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo have developed a way to ramp up the conversion of skin cells into neurons that can produce dopamine.
For decades, the elusive holy grail in Parkinson’s disease research has been finding a way to repair faulty dopamine neurons and put them back into patients, where they will start producing dopamine again. Researchers have tried fetal material, which is difficult to obtain and of variable quality, and embryonic stem cells (a long process with a low yield), and more recently, skin cells (difficult to obtain sufficient quantities of neurons).
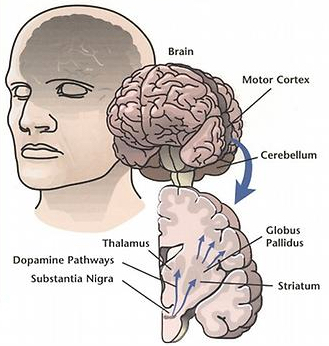
To control movement and balance, dopamine signals travel from the substantia nigra in the midbrain up to brain regions including the corpus striatum, the globus pallidus, and the thalamus. But in Parkinson’s disease, most of the dopamine signals from the substantia nigra are lost. (credit: NIH)
Bypassing the cellular “gatekeeper”
The new UB research, published Dec. 7 in an open-access article in Nature Communications, is based on their discovery that p53, a transcription factor protein, acts as a gatekeeper protein.
“We found that p53 tries to maintain the status quo in a cell; it guards against changes from one cell type to another,” explained Jian Feng, PhD, senior author and professor in the Department of Physiology and Biophysics in the Jacobs School of Medicine and Biomedical Sciences at UB.
This is, p53 acts as a kind of gatekeeper protein to prevent conversion into another type of cell. “Once we lowered the expression of p53, then things got interesting: We were able to reprogram the [skin cell] fibroblasts into neurons much more easily.”
The advance may also be important for basic cell biology, Feng said. “This is a generic way for us to change cells from one type to another,” he said. “It proves that we can treat the cell as a software system when we remove the barriers to change. If we can identify transcription factor combinations that control which genes are turned on and off, we can change how the genome is being read. We might be able to play with the system more quickly and we might be able to generate tissues similar to those in the body, even brain tissue.
“People like to think that things proceed in a hierarchical way, that we start from a single cell and develop into an adult with about 40 trillion cells, but our results prove that there is no hierarchy,” he continued. “All our cells have the same source code as our first cell; this code is read differently to generate all types of cells that make up the body.”
Generating new dopamine neurons via cellular conversion
Timing was key to their success. “We found that the point in the cell cycle just before the cell tries to sense its environment to ensure that all is ready for duplicating the genome is the prime time when the cell is receptive to change,” said Feng.
By lowering the genomic gatekeeper p53 at the right time of cell cycle, they could easily turn the skin cells into dopamine neurons, using transcription-factor combinations discovered in previous studies. These manipulations turn on the expression of Tet1, a DNA modification enzyme that changes how the genome is read.
“Our method is faster and much more efficient than previously developed ones,” said Feng. “The best previous method could take two weeks to produce 5 percent dopamine neurons. With ours, we got 60 percent dopamine neurons in ten days.”
The researchers have done multiple experiments to prove that these neurons are functional mid-brain dopaminergic neurons, the type lost in Parkinson’s disease.
The finding may enable researchers to generate patient-specific neurons in a dish that could then be transplanted into the brain to repair the faulty neurons, or used to efficiently screen new treatments for Parkinson’s disease.
Abstract of Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons
The direct conversion of fibroblasts to induced dopaminergic (iDA) neurons and other cell types demonstrates the plasticity of cell fate. The low efficiency of these relatively fast conversions suggests that kinetic barriers exist to safeguard cell-type identity. Here we show that suppression of p53, in conjunction with cell cycle arrest at G1 and appropriate extracellular environment, markedly increase the efficiency in the transdifferentiation of human fibroblasts to iDA neurons by Ascl1, Nurr1, Lmx1a and miR124. The conversion is dependent on Tet1, as G1 arrest, p53 knockdown or expression of the reprogramming factors induces Tet1 synergistically. Tet1 knockdown abolishes the transdifferentiation while its overexpression enhances the conversion. The iDA neurons express markers for midbrain DA neurons and have active dopaminergic transmission. Our results suggest that overcoming these kinetic barriers may enable highly efficient epigenetic reprogramming in general and will generate patient-specific midbrain DA neurons for Parkinson’s disease research and therapy.