‘Perfect’ low-cost, defect-free graphene directly from graphite
September 15, 2016
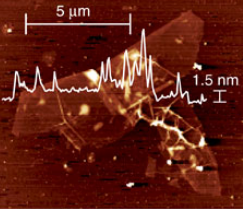
Atomic force microscope (AFM) image with height profile indicating the single-layer nature of the obtained graphene, with lateral dimensions of ~10 micrometers and a height of ~1.5 nanometers. (credit: Philipp Vecera et al./Nature Communications)
Chemists at the University of Erlangen-Nürnberg (FAU) in Germany and the University of Vienna have succeeded in producing “perfect” defect-free, high-quality graphene directly from graphite (“pencil lead”) for the first time. This new low-cost method may make it possible for the semiconductor industry to scale up use of graphene in pioneering technologies such as transparent electrodes for flexible displays.
The chemists say their method enables the graphene to be cut without causing defects and allows specific electronic properties to be set. That makes this new scalable, inexpensive method for graphene production a significant improvement over previous wet-chemical approaches, which have size limitations and excessive defects, lowering the conductivity .
Graphene is usually processed using chemical exfoliation (peeling off) of graphite. In this process, metal ions are embedded in graphite, resulting in an intercalated (layered) compound. The individual layers of carbon are separated using solvents. The stabilized graphene then has to be separated from the solvent and reoxidised. However, defects in the individual layers of carbon, such as hydration and oxidation of carbon atoms in the lattice, can occur during this process.
FAU researchers have now found a solution to this problem: adding the solvent benzonitrile, which allows the defect-free graphene to be removed without any additional functional groups forming.
In addition, the benzonitrile (PhCN) molecule formed during the reduction reaction turns red. This change in color allows the number of charge carriers in the system to be determined easily through absorption measurements instead of requiring complex voltage measurements.
The researchers published their findings August 10 in the open-access journal Nature Communications.
Abstract of Solvent-driven electron trapping and mass transport in reduced graphites to access perfect graphene
Herein, we report on a significant discovery, namely, the quantitative discharging of reduced graphite forms, such as graphite intercalation compounds, graphenide dispersions and graphenides deposited on surfaces with the simple solvent benzonitrile. Because of its comparatively low reduction potential, benzonitrile is reduced during this process to the radical anion, which exhibits a red colour and serves as a reporter molecule for the quantitative determination of negative charges on the carbon sheets. Moreover, this discovery reveals a very fundamental physical–chemical phenomenon, namely a quantitative solvent reduction induced and electrostatically driven mass transport of K+ ions from the graphite intercalation compounds into the liquid. The simple treatment of dispersed graphenides suspended on silica substrates with benzonitrile leads to the clean conversion to graphene. This unprecedented procedure represents a rather mild, scalable and inexpensive method for graphene production surpassing previous wet-chemical approaches.