Pulsed laser light turns whole-brain activity on and off
December 18, 2015
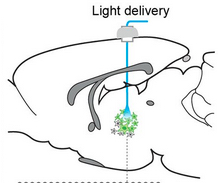
Optogenetic laser light stimulation of the thalamus (credit: Jia Liu et al./eLife)
By flashing high-frequency (40 to 100 pulses per second) optogenetic lasers at the brain’s thalamus, scientists were able to wake up sleeping rats and cause widespread brain activity. In contrast, flashing the laser at 10 pulses per second suppressed the activity of the brain’s sensory cortex and caused rats to enter a seizure-like state of unconsciousness.
“We hope to use this knowledge to develop better treatments for brain injuries and other neurological disorders,” said Jin Hyung Lee, Ph.D., assistant professor of neurology, neurosurgery, and bioengineering at Stanford University, and a senior author of the study, published in the open-access journal eLIFE.
Located deep inside the brain, the thalamus regulates arousal, acting as a relay station to the cortex for neural signals from the body. Damage to neurons in the central part of the thalamus may lead to problems with sleep, attention, and memory.*
Combining light stimulation and fMRI measurements
The observations used a combination of optogenetics and whole-brain functional MRI (fMRI) — known as “ofMRI” — to detect overall effects on the brain, along with EEG and single-unit cell recordings.The researchers noted in the paper that “using targeted, temporally precise optogenetic stimulation in the current study allowed us to selectively excite a single group of neuronal elements and identify their specific role in creating distinct modes of network function.” That could not be achieved with conventional electrode stimulation, the researchers say.
They explain that this method may allow for direct-brain stimulation (DBS) therapeutic methods to be optimized in the clinic “for a wide range of neurological disorders that currently lack such treatment.”
“This study takes a big step towards understanding the brain circuitry that controls sleep and arousal,” Yejun (Janet) He, Ph.D., program director at NIH’s National Institute of Neurological Disorders and Stroke (NINDS), which partially funded the study.
* Further experiments suggested the different effects may be due to a unique firing pattern by inhibitory neurons in a neighboring brain region, the zona incerta, during low frequency stimulation. Cells in this brain region have been shown to send inhibitory signals to cells in the sensory cortex. Electrical recordings showed that during low frequency stimulation of the central thalamus, zona incerta neurons fired in a spindle pattern that often occurs during sleep. In contrast, sleep spindles did not occur during high frequency stimulation. Moreover, when the scientists blocked the firing of the zona incerta neurons during low frequency stimulation of the central thalamus, the average activity of sensory cortex cells increased.
Abstract of Frequency-selective control of cortical and subcortical networks by central thalamus
Central thalamus plays a critical role in forebrain arousal and organized behavior. However, network-level mechanisms that link its activity to brain state remain enigmatic. Here, we combined optogenetics, fMRI, electrophysiology, and video-EEG monitoring to characterize the central thalamus-driven global brain networks responsible for switching brain state. 40 and 100 Hz stimulations of central thalamus caused widespread activation of forebrain, including frontal cortex, sensorimotor cortex, and striatum, and transitioned the brain to a state of arousal in asleep rats. In contrast, 10 Hz stimulation evoked significantly less activation of forebrain, inhibition of sensory cortex, and behavioral arrest. To investigate possible mechanisms underlying the frequency-dependent cortical inhibition, we performed recordings in zona incerta, where 10, but not 40, Hz stimulation evoked spindle-like oscillations. Importantly, suppressing incertal activity during 10 Hz central thalamus stimulation reduced the evoked cortical inhibition. These findings identify key brain-wide dynamics underlying central thalamus arousal regulation.