Real-time noninvasive PET imaging to detect tumors
May 4, 2015
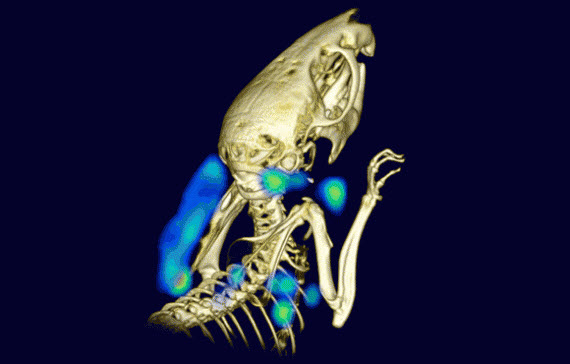
Non-invasive PET imaging with single-domain antibodies detects lymphoid organs and inflammation. Here, symmetrical lymph nodes and tumor-associated cells are visible in a living mouse that was inoculated subcutaneously with human melanoma cells on the back of the left shoulder. (credit: Mohammad Rashidian et al/PNAS)
Whitehead Institute scientists have developed a method to detect tumors by real-time imaging of the immune system using positron emission tomography (PET). The new method replaces blood draws and invasive biopsies. It’s a potential breakthrough in diagnostics and monitoring the efficacy of cancer therapies.
An example of such a therapy is a new way to get the entire immune system to attack cancer, which KurzweilAI recently reported. The new PET imaging method now allows for monitoring the effects of such treatments. To develop the method, Whitehead Institute member Hidde Ploegh leveraged two research tools:
- Single-domain antibodies known as VHHs, derived from the heavy chain-only antibodies made by the immune systems of animals in the camelid family (such as camels, llamas, and alpacas). Ploegh’s lab immunizes alpacas to generate VHHs specific to immune cells of interest.
- Sortagging, which labels the VHHs in a site-specific fashion to enable tracking of the VHHs and their targets in a living animal.
Immune cells pinpoint tumor locations
The tumor giveaway is found in the tissue around tumors. The tissue often contains immune cells such as neutrophils (discussed in the KurzweilAI article) and macrophages. So Ploegh and his lab members hypothesized that appropriately labeled VHHs might allow them to pinpoint tumor locations by finding the tumor-associated immune cells.
VHHs’ extremely small size — approximately one-tenth that of conventional antibodies — are likely responsible for their superior tissue penetration and thus makes them particularly well suited for such use, says Ploegh.
His lab generated VHHs that recognize mouse immune cells, labeled these VHHs with radioisotopes, and injected them into tumor-bearing mice. Then they used PET imaging, which detected the location of immune cells around the tumor quickly and accurately.
“We were able to image tumors as small as one millimeter in size and within just a few days of their starting to grow,” says Mohammad Rashidian, a postdoctoral researcher in Ploegh’s lab and first author of the PNAS paper. “We’re very excited about this because it’s a powerful approach to pick up inflammation in and around the tumor.”
Monitoring cancer immunotherapy
Rashidian and Ploegh believe that with further refinement, the method could be used to monitor response to — and perhaps modify — cancer immunotherapy.
“To succeed with immunotherapy, we need more information about the tumor microenvironment,” says Rashidian. “With this method, you could perhaps start immunotherapy, and then, a few weeks later, image with VHHs to figure out progress and success of treatment.”
“PET imaging should allow a much more comprehensive look at the entire tumor in its environment,” says Ploegh. Then we can ask, ‘Did the tumor grow? Did immune cells invade? What has happened to the tumor?’ And to be able to see this without going in invasively is a significant achievement.”
This work is supported by the National Institutes of Health, the Cancer Research Institute, the Calouste Gulbenkian Foundation, the Champalimaud Foundation, the Portuguese Science and Technology Foundation, the Portuguese Ministry of Health, and the Lustgarten Foundation.
Abstract of Noninvasive imaging of immune response
At their margins, tumors often contain neutrophils, dendritic cells, and activated macrophages, which express class II MHC and CD11b products. The interplay between stromal cells, tumor cells, and migratory cells such as lymphocytes creates opportunities for noninvasive imaging of immune responses. We developed alpacaderived antibody fragments specific for mouse class II MHC and CD11b products, expressed on the surface of a variety ofmyeloid cells. We validated these reagents by flow cytometry and two-photon microscopy to obtain images at cellular resolution. To enable noninvasive imaging of the targeted cell populations, we developed a method to site-specifically label VHHs [the variable domain (VH) of a camelid heavy-chain only antibody] with 18F or 64Cu. Radiolabeled VHHs rapidly cleared the circulation (t1/2 ≈ 20 min) and clearly visualized lymphoid organs. We used VHHs to explore the possibility of imaging inflammation in both xenogeneic and syngeneic tumor models, which resulted in detection of tumors with remarkable specificity. We also imaged the infiltration of myeloid cells upon injection of complete Freund’s adjuvant. Both anti-class II MHC and anti- CD11b VHHs detected inflammation with excellent specificity. Given the ease of manufacture and labeling of VHHs, we believe that this method could transform the manner in which antitumor responses and/or infectious events may be tracked.