Researchers use optogenetic light to block tumor development
March 28, 2016
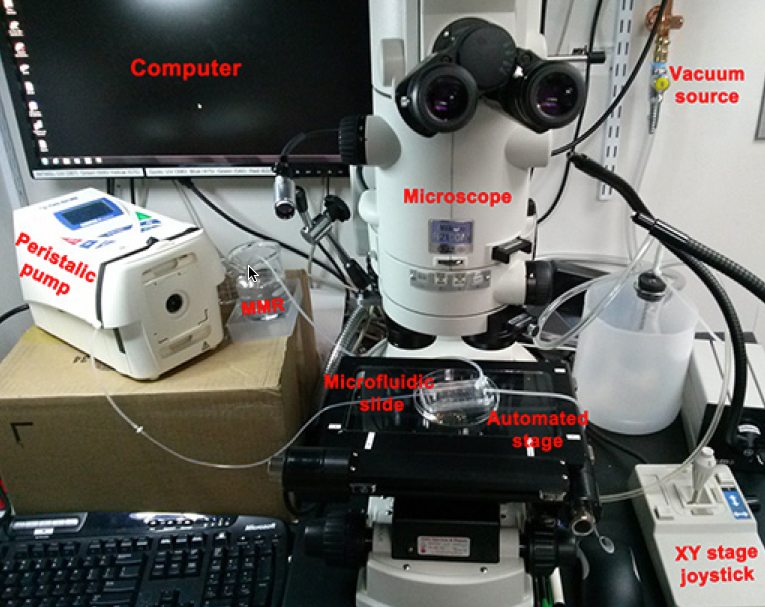
Setup for delivering spatio-temporally precise light stimulation of optogenetic proteins expressed in tadpole embryo induced tumor-like structures. (credit: Brook T. Chernet et al./Oncotarget)
Tufts University biologists have demonstrated (using a frog model*) for the first time that it is possible to prevent tumors from forming (and to normalize tumors after they have formed) by using optogenetics (light) to control bioelectrical signalling among cells.
Light/bioelectric control of tumors
Virtually all healthy cells maintain a more negative voltage in the cell interior compared with the cell exterior. But the opening and closing of ion channels in the cell membrane can cause the voltage to become more positive (depolarizing the cell) or more negative (polarizing the cell). That makes it possible to detect tumors by their abnormal bioelectrical signature before they are otherwise apparent.
The study was published online in an open-access paper in Oncotarget on March 16.
The use of light to control ion channels has been a ground-breaking tool in research on the nervous system and brain, but optogenetics had not yet been applied to cancer.
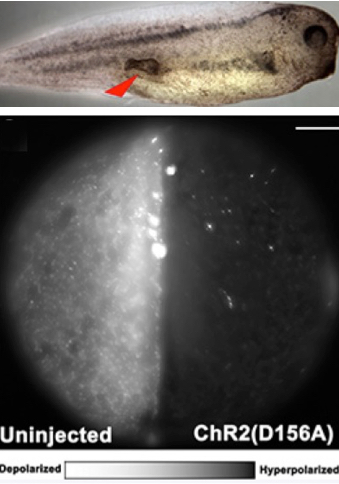
Optogenetics modulation of membrane voltage to control induced tumor-like structures. (Top) Tumor induced in tadpole embryo. (Bottom left) Control embryo not injected with light-sensitive protein is highly fluorescent, indicating relative depolarization. (Bottom right) Embryo injected with light-sensitive protein exhibits hyperpolarization, significantly lowering the incidence of tumor formation. Scale bar = 150 micrometers. (credit: Brook T. Chernet et al./Oncotarget)
The researchers first injected cells in Xenopus laevis (frog) embryos with RNA that encoded a mutant RAS oncogene known to cause cancer-like growths.
The researchers then used blue light to activate positively charged ion channels,which induced an electric current that caused the cells to go from a cancer-like depolarized state to a normal, more negative polarized state. The did the same with a green light-activated proton pump, Archaerhodopsin (Arch). Activation of both agents significantly lowered the incidence of tumor formation and also increased the frequency with which tumors regressed into normal tissue.
“These electrical properties are not merely byproducts of oncogenic processes. They actively regulate the deviations of cells from their normal anatomical roles towards tumor growth and metastatic spread,” said senior and corresponding author Michael Levin, Ph.D., who holds the Vannevar Bush chair in biology and directs the Center for Regenerative and Developmental Biology at Tufts School of Arts and Sciences.
“Discovering new ways to specifically control this bioelectrical signaling could be an important path towards new biomedical approaches to cancer. This provides proof of principle for a novel class of therapies which use light to override the action of oncogenic mutations,” said Levin. “Using light to specifically target tumors would avoid subjecting the whole body to toxic chemotherapy or similar reagents.”
This work was supported by the G. Harold and Leila Y. Mathers Charitable Foundation.
* Frogs are a good model for basic science research into cancer because tumors in frogs and mammals share many of the same characteristics. These include rapid cell division, tissue disorganization, increased vascular growth, invasiveness and cells that have an abnormally positive internal electric voltage.
Abstract of Use of genetically encoded, light-gated ion translocators to control tumorigenesis
It has long been known that the resting potential of tumor cells is depolarized relative to their normal counterparts. More recent work has provided evidence that resting potential is not just a readout of cell state: it regulates cell behavior as well. Thus, the ability to control resting potential in vivo would provide a powerful new tool for the study and treatment of tumors, a tool capable of revealing living-state physiological information impossible to obtain using molecular tools applied to isolated cell components. Here we describe the first use of optogenetics to manipulate ion-flux mediated regulation of membrane potential specifically to prevent and cause regression of oncogene-induced tumors. Injection of mutant-KRAS mRNA induces tumor-like structures with many documented similarities to tumors, in Xenopus tadpoles. We show that expression and activation of either ChR2D156A, a blue-light activated cation channel, or Arch, a green-light activated proton pump, both of which hyperpolarize cells, significantly lowers the incidence of KRAS tumor formation. Excitingly, we also demonstrate that activation of co-expressed light-activated ion translocators after tumor formation significantly increases the frequency with which the tumors regress in a process called normalization. These data demonstrate an optogenetic approach to dissect the biophysics of cancer. Moreover, they provide proof-of-principle for a novel class of interventions, directed at regulating cell state by targeting physiological regulators that can over-ride the presence of mutations.