Researchers regrow hair, cartilage, bone, soft tissues
November 12, 2013
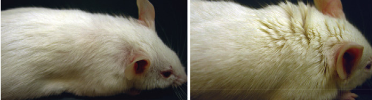
Hair Club for Mice: a reactivated dormant gene called Lin28 stimulated hair growth in mouse on the right, compared to non-treated mouse (left) (credit: Ng Shyh-Chang et al./Cell)
Young animals are known to repair their tissues effortlessly, but can this capacity be recaptured in adults? A new study from researchers at the Stem Cell Program at Boston Children’s Hospital suggests that it can.
By reactivating a dormant gene called Lin28a, which is active in embryonic stem cells, researchers were able to regrow hair and repair cartilage, bone, skin and other soft tissues in a mouse model.
The study also found that Lin28a promotes tissue repair in part by enhancing metabolism in mitochondria — the energy-producing engines in cells — suggesting that a mundane cellular “housekeeping” function could open new avenues for developing regenerative treatments. `
“Efforts to improve wound healing and tissue repair have mostly failed, but altering metabolism provides a new strategy which we hope will prove successful,” says the study’s senior investigator George Q. Daley, MD, PhD, director of Boston Children’s Stem Cell Transplantation Program and an investigator with the Howard Hughes Medical Institute.
“Most people would naturally think that growth factors are the major players in wound healing, but we found that the core metabolism of cells is rate-limiting in terms of tissue repair,” adds PhD candidate Shyh-Chang Ng, co-first author on the paper with Hao Zhu, MD, both scientists in the Daley Lab. “The enhanced metabolic rate we saw when we reactivated Lin28a is typical of embryos during their rapid growth phase.”
Lin28, first discovered in worms, functions in all complex organisms. It is abundant in embryonic stem cells, expressed strongly during early embryo formation and has been used to reprogram skin cells into stem cells. It acts by binding to RNA and regulating how genes are translated into proteins.
Stimulating tissue growth via mitochrondria
To better understand how Lin28a promotes tissue repair, the researchers systematically looked at what specific RNAs it binds to. They found that Lin28a also enhances the production of metabolic enzymes in mitochondria, the structures that produce energy for the cell. By revving up a cell’s bioenergetics, they found, Lin28a helps generate the energy needed to stimulate and grow new tissues.
“We already know that accumulated defects in mitochondrial metabolism can lead to aging in many cells and tissues,” says Shyh-Chang. “We are showing the converse — that enhancement of mitochondrial metabolism can boost tissue repair and regeneration, recapturing the remarkable repair capacity of juvenile animals.”
Further experiments showed that bypassing Lin28a and directly activating mitochondrial metabolism with a small-molecule compound also had the effect of enhancing wound healing. This suggests the possibility of inducing regeneration and promoting tissue repair with drugs.
“Since Lin28 itself is difficult to introduce into cells, the fact that we were able to activate mitochondrial metabolism pharmacologically gives us hope,” Shyh-Chang says.
Lin28A didn’t universally induce regeneration in all tissues. Heart tissue showed little effect, and while the researchers were able to enhance the regrowth of finger tips in newborn mice, they could not in adults.
“Lin28a could be a key factor in constituting a healing cocktail,” says Shyh-Chang, “but there are other embryonic factors that remain to be found.”
The study was supported by an A*STAR National Science Scholarship, a Graduate Training in Cancer Research Grant, an American Cancer Society Postdoctoral Fellowship, a National Institutes of Health K08 grant, CPRIT, a Herchel Smith Graduate Fellowship, the Ellison Medical Foundation, the Howard Hughes Medical Institute and the Manton Center for Orphan Disease Research.
Abstract of Cell paper
Regeneration capacity declines with age, but why juvenile organisms show enhanced tissue repair remains unexplained. Lin28a, a highly conserved RNA-binding protein expressed during embryogenesis, plays roles in development, pluripotency, and metabolism. To determine whether Lin28a might influence tissue repair in adults, we engineered the reactivation of Lin28a expression in several models of tissue injury. Lin28a reactivation improved hair regrowth by promoting anagen in hair follicles and accelerated regrowth of cartilage, bone, and mesenchyme after ear and digit injuries. Lin28a inhibits let-7 microRNA biogenesis; however, let-7 repression was necessary but insufficient to enhance repair. Lin28a bound to and enhanced the translation of mRNAs for several metabolic enzymes, thereby increasing glycolysis and oxidative phosphorylation (OxPhos). Lin28a-mediated enhancement of tissue repair was negated by OxPhos inhibition, whereas a pharmacologically induced increase in OxPhos enhanced repair. Thus, Lin28a enhances tissue repair in some adult tissues by reprogramming cellular bioenergetics.