Researchers regrow human corneas in mice
July 7, 2014
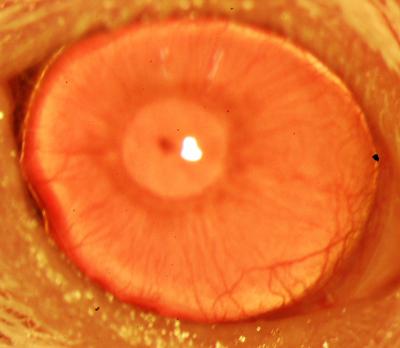
A restored functional cornea following transplantation of human ABCB5-positive limbal stem cells to limbal stem cell-deficient mice (credit: Kira Lathrop, Bruce Ksander, Markus Frank, and Natasha Frank)
A team of Boston medical researchers has identified a way to trigger regrowth of human corneal tissue using stem cells. The finding could restore vision for victims of chemical injury and others with damaging eye diseases.
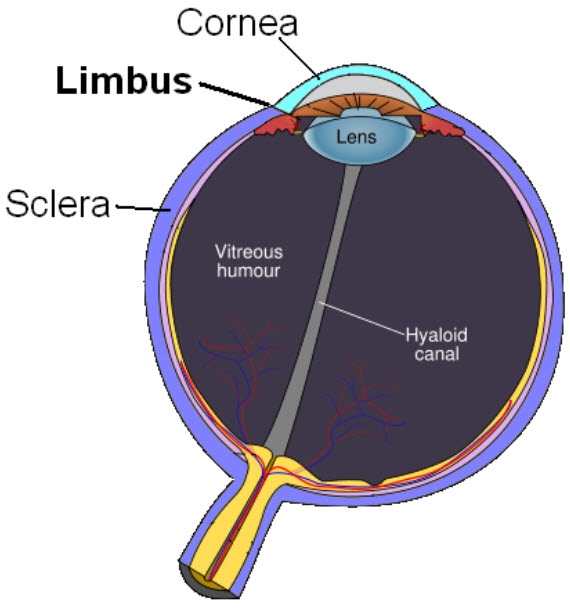
The corneal limbus, a source of stem cells, is the border of the cornea and the sclera (the white of the eye) (credit: Wikimedia Commons)
Limbal stem cells, which reside in the eye’s limbus, help maintain and regenerate corneal tissue. Their loss due to injury or disease is one of the leading causes of blindness.
In the past, tissue or cell transplants have been used to help the cornea regenerate, but it was unknown whether there were actual limbal stem cells in the grafts, or how many, and the outcomes were not consistent.
A marker for limbal stem cells
In this new study, researchers at the Massachusetts Eye and Ear/Schepens Eye Research Institute (Mass. Eye and Ear), Boston Children’s Hospital, Brigham and Women’s Hospital, and the VA Boston Healthcare System used a molecule known as ABCB5, which acts as a marker for hard-to-find limbal stem cells.
ABCB5 allowed the researchers to locate hard-to-find limbal stem cells in tissue from deceased human donors and use these stem cells to regrow anatomically correct, fully functional human corneas in mice.
“Limbal stem cells are very rare, and successful transplants are dependent on these rare cells,” says Bruce Ksander, Ph.D., of Mass. Eye and Ear, co-lead author on the study with post-doctoral fellow Paraskevi Kolovou, M.D. “This finding will now make it much easier to restore the corneal surface. It’s a very good example of basic research moving quickly to a translational application.”
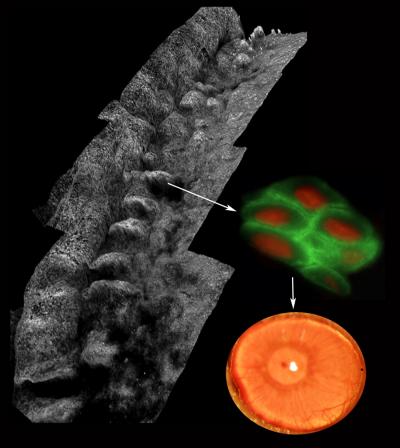
Composite image depicting the palisades of Vogt within the human limbus (left), ABCB5-positive limbal stem cells isolated from the palisades (right; ABCB5 — green, nucleus — red) and a restored functional cornea following transplantation of human ABCB5-positive limbal stem cells to limbal stem cell-deficient mice (bottom right) (credit: Kira Lathrop, Bruce Ksander, Markus Frank, and Natasha Frank)
The research, published in Nature, is also one of the first known examples of constructing a tissue from an adult-derived human stem cell, the researchers say.
The discovery
ABCB5 was originally discovered in the lab of Markus Frank, M.D., of Boston Children’s Hospital, and Natasha Frank, M.D., of the VA Boston Healthcare System and Brigham and Women’s Hospital (co-senior investigators on the study) as being produced in tissue precursor cells in human skin and intestine.
In the new work, using a mouse model developed by the Frank lab, they found that ABCB5 also occurs in limbal stem cells and is required for their maintenance and survival, and for corneal development and repair. Mice lacking a functional ABCB5 gene lost their populations of limbal stem cells, and their corneas healed poorly after injury.
“ABCB5 allows limbal stem cells to survive, protecting them from apoptosis [programmed cell death],” says Markus Frank. “The mouse model allowed us for the first time to understand the role of ABCB5 in normal development, and should be very important to the stem cell field in general.” according to Natasha Frank.
Markus Frank is working with the biopharmaceutical industry to develop a clinical-grade ABCB5 antibody that would meet U.S. regulatory approvals.
“A single lab cannot do a study like this,” says Natasha Frank, also affiliated with the Harvard Stem Cell Institute. “It integrates genetics, knockout mice, antibodies, transplantation—a lot of technical expertise that we were lucky came together in a very nice way.
Human trials planned
“Our study demonstrated that the cell surface protein ABCB5 is essential for normal limbal stem cell function in corneal development and repair, through critical roles in stem cell maintenance and survival,” she explained to KurzweilAI in an email. “Furthermore, our findings show that the capacity to fully restore the cornea is exclusively contained within the ABCB5-positive limbal stem cell compartment (human or mouse), indicating that this pure limbal stem cell population has the potential to significantly improve therapy for corneal disease associated with limbal stem cell deficiency.
“Until now, no molecular marker existed for limbal stem cells by which these rare cells could be isolated and purified. Previously published work on limbal epithelial cell grafts showed that when more than three percent of transplanted cells were stem cells, transplants were successful — less than three percent and the transplants were not. We hypothesized that ABCB5 would represent a marker for limbal stem cells, based on its expression on stem and progenitor cell populations in other human tissues.
“While we tested the capacity of human limbal stem cells to regenerate the cornea upon grafting to limbal stem cell-deficient mice, we would expect this novel transplant approach to also work in humans, and efforts are currently underway to meet regulatory requirements to allow testing in human clinical trials.
“More research to develop the technique and make sure it is safe will be required before human trials can take place, and we expect this to take one to two years before entering clinical trials, with several additional years of clinical study needed before the treatment might become ultimately available.”
The research was supported by the National Institute of Neurological Disorders and Stroke (grant K08NS051349), the Veterans Administration (BLR&D 1I01BX000516 and VA RR&D 1I01RX000989), the Harvard Stem Cell Institute, the National Cancer Institute (R01CA113796, R01CA158467, R01CA138231), the Department of Defense (PR0332453), the National Institutes of Health (R01EY018624, P30EY014801, R01EY021768, R01CA138231, R01EB017274, U01HL100402, P41EB015903 and NIH New Innovator Award DP2OD007483), the Corley Research Foundation, the Western Pennsylvania Medical Eye Bank Core Grant for Vision Research (EY08098), the Howard Hughes Medical Institute and the Life Sciences Research Foundation.
Abstract of Nature paper
Corneal epithelial homeostasis and regeneration are sustained by limbal stem cells (LSCs), and LSC deficiency is a major cause of blindness worldwide. Transplantation is often the only therapeutic option available to patients with LSC deficiency. However, while transplant success depends foremost on LSC frequency within grafts, a gene allowing for prospective LSC enrichment has not been identified so far. Here we show that ATP-binding cassette, sub-family B, member 5 (ABCB5) marks LSCs and is required for LSC maintenance, corneal development and repair. Furthermore, we demonstrate that prospectively isolated human or murine ABCB5-positive LSCs possess the exclusive capacity to fully restore the cornea upon grafting to LSC-deficient mice in xenogeneic or syngeneic transplantation models. ABCB5 is preferentially expressed on label-retaining LSCs in mice and p63α-positive LSCs in humans. Consistent with these findings, ABCB5-positive LSC frequency is reduced in LSC-deficient patients. Abcb5 loss of function in Abcb5 knockout mice causes depletion of quiescent LSCs due to enhanced proliferation and apoptosis, and results in defective corneal differentiation and wound healing. Our results from gene knockout studies, LSC tracing and transplantation models, as well as phenotypic and functional analyses of human biopsy specimens, provide converging lines of evidence that ABCB5 identifies mammalian LSCs. Identification and prospective isolation of molecularly defined LSCs with essential functions in corneal development and repair has important implications for the treatment of corneal disease, particularly corneal blindness due to LSC deficiency.