Scientists digitally mimic evolution to create novel proteins
May 10, 2016
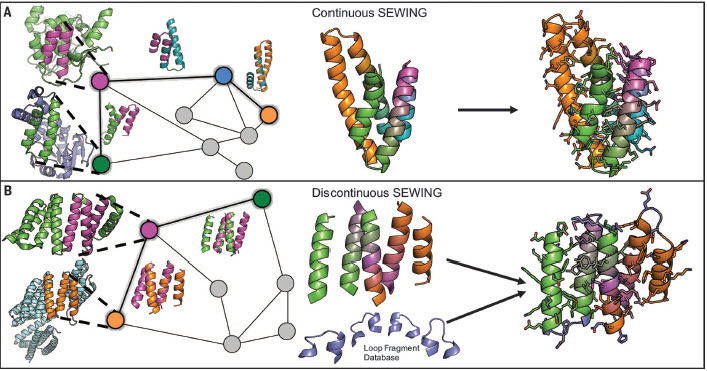
Overview of the SEWING method. (A) Continuous SEWING workflow. (B) Discontinuous SEWING workflow. Left: Parental Protein Data Banks are shown with extracted substructures; graph schematic — colored nodes indicate substructures contained in the final design model, and superimposed structures show structural similarity indicated by adjacent edges. Center: design model before sequence optimization and loop design. Right: final design models. (credit: T. M. Jacobs et al./Science)
Here’s an innovative idea: create new proteins by simply “sewing” together pieces of existing proteins. That’s exactly what researchers at the University of North Carolina School of Medicine have done to design new “cellular machines” needed to understand and battle diseases.
Published today in the journal Science, the new technique, called SEWING, was inspired by natural evolutionary mechanisms that also recombine portions of the 100,000 different known proteins in the body.
First, throw out the blueprint
Traditionally, researchers have used computational protein design to recreate in the laboratory what already exists in the natural world. But in recent years, their focus has shifted toward inventing novel proteins with new functionality. Those design projects all start with a specific structural “blueprint” in mind, and as a result are limited.
Senior study author Brian Kuhlman, PhD, professor of biochemistry and biophysics, and his colleagues believe that by removing the limitations of a pre-determined blueprint and taking cues from evolution, they can more easily create new functional proteins.
“We can now begin to think about engineering proteins to do things that nothing else is capable of doing,” he said. “The structure of a protein determines its function, so if we are going to learn how to design new functions, we have to learn how to design new structures. Our study is a critical step in that direction and provides tools for creating proteins that haven’t been seen before in nature.”
Mimicking nature
To mimic the mechanisms of natural protein evolution, they developed a computer design strategy called SEWING (Structure Extension With Native-substructure Graphs):
- The researchers digitally chopped up naturally occurring proteins into well-defined pieces.
- They performed a series of computational tests to figure out which pieces would fit well together. In nature, this step would involve looking for stretches of amino acid sequences that are similar between proteins. On the computer, it involved searching for regions of structural similarity to make things fit in the right place.
- They whittled down the list to the top 21 proteins, which they produced in the lab.
- To confirm what they created, they took pictures of these proteins using x-ray crystallography and nuclear magnetic resonance (NMR), and found that the proteins contained all the unique structural varieties they had designed on the computer.
Currently, the researchers are using SEWING to create proteins that can bind to several other proteins at a time. Many of the most important natural proteins are similar multitaskers, including the blood protein hemoglobin, which carries four copies of oxygen from the lungs to the body’s tissues.
Abstract of Design of structurally distinct proteins using strategies inspired by evolution
Natural recombination combines pieces of preexisting proteins to create new tertiary structures and functions. We describe a computational protocol, called SEWING, which is inspired by this process and builds new proteins from connected or disconnected pieces of existing structures. Helical proteins designed with SEWING contain structural features absent from other de novo designed proteins and, in some cases, remain folded at more than 100°C. High-resolution structures of the designed proteins CA01 and DA05R1 were solved by x-ray crystallography (2.2 angstrom resolution) and nuclear magnetic resonance, respectively, and there was excellent agreement with the design models. This method provides a new strategy to rapidly create large numbers of diverse and designable protein scaffolds.