Scientists grow beating heart tissue on spinach leaves
March 31, 2017
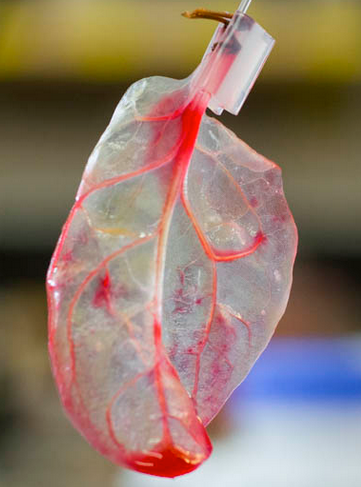
(credit: Worcester Polytechnic Institute)
A research team headed by Worcester Polytechnic Institute (WPI) scientists* has solved a major tissue engineering problem holding back the regeneration of damaged human tissues and organs: how to grow small, delicate blood vessels, which are beyond the capabilities of 3D printing.**
The researchers used plant leaves as scaffolds (structures) in an attempt to create the branching network of blood vessels — down to the capillary scale — required to deliver the oxygen, nutrients, and essential molecules required for proper tissue growth.
In a series of unconventional experiments, the team cultured beating human heart cells on spinach leaves that were stripped of plant cells.*** The researchers first decellularized spinach leaves (removed cells, leaving only the veins) by perfusing (flowing) a detergent solution through the leaves’ veins. What remained was a framework made up primarily of biocompatible cellulose, which is already used in a wide variety of regenerative medicine applications, such as cartilage tissue engineering, bone tissue engineering, and wound healing.

A spinach leaf (left) was decellularized in 7 days, leaving only the scaffold (right), which served as an intact vascular network. As a test, red dye was pumped through its veins, simulating blood, oxygen, and nutrients. Cardiomyocytes (cardiac muscle cells) derived from human pluripotent stem cells were then seeded onto the surface of the leaf scaffold, forming cell clusters that demonstrated cardiac contractile function and calcium-handling capabilities for 21 days. (credit: Worcester Polytechnic Institute)
After testing the spinach vascular (leaf vessel structure) system mechanically by flowing fluids and microbeads similar in size to human blood cells through it, the researchers seeded the vasculature with human umbilical vein endothelial cells (HUVECs) to grow endothelial cells (which line blood vessels).
Human mesenchymal stem cells (hMSC) and human pluripotent stem-cell-derived cardiomyocytes (cardiac muscle cells) (hPS-CM) were then seeded to the outer surfaces of the plant scaffolds. The cardiomyocytes spontaneously demonstrated cardiac contractile function (beating) and calcium-handling capabilities over the course of 21 days.
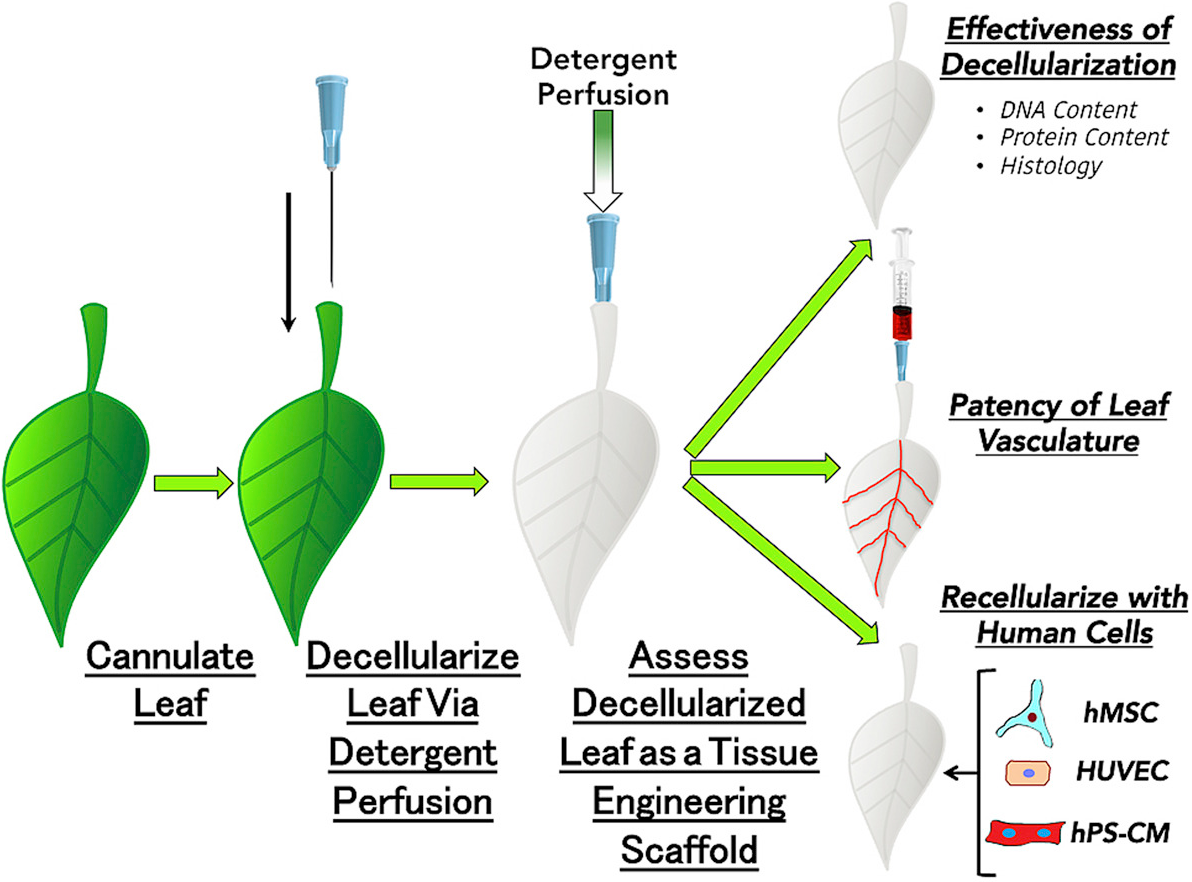
The decellurize-recellurize process (credit: Joshua R. Gershlak et al./Biomaterials)
The future of “crossing kingdoms”
These proof-of-concept studies may open the door to using multiple spinach leaves to grow layers of healthy heart muscle, and a potential tissue engineered graft based upon the plant scaffolds could use multiple leaves, where some act as arterial support and some act as venous return of blood and fluids from human tissue, say the researchers.
“Our goal is always to develop new therapies that can treat myocardial infarction, or heart attacks,” said Glenn Gaudette, PhD, professor of biomedical engineering at WPI and corresponding author of an open-access paper in the journal Biomaterials, published online in advance of the May 2017 issue.
“Unfortunately, we are not doing a very good job of treating them today. We need to improve that. We have a lot more work to do, but so far this is very promising.”
Currently, it’s not clear how the plant vasculature would be integrated into the native human vasculature and whether there would be an immune response, the authors advise.
The researchers are also now optimizing the decellularization process and seeing how well various human cell types grow while they are attached to (and potentially nourished by) various plant-based scaffolds that could be adapted for specialized tissue regeneration studies. “The cylindrical hollow structure of the stem of Impatiens capensis might better suit an arterial graft,” the authors note. “Conversely, the vascular columns of wood might be useful in bone engineering due to their relative strength and geometries.”
Other types of plants could also provide the framework for a wide range of other tissue engineering technologies, the authors suggest.****
The authors conclude that “development of decellularized plants for scaffolding opens up the potential for a new branch of science that investigates the mimicry between kingdoms, e.g., between plant and animal. Although further investigation is needed to understand future applications of this new technology, we believe it has the potential to develop into a ‘green’ solution pertinent to a myriad of regenerative medicine applications.”
* The research team also includes human stem cell and plant biology researchers at the University of Wisconsin-Madison, and Arkansas State University-Jonesboro.
** The research is driven by the pressing need for organs and tissues available for transplantation, which far exceeds their availability. More than 100,000 patients are on the donor waiting list at any given time and an average of 22 people die each day while waiting for a donor organ or tissue to become available, according to a 2016 paper in the American Journal of Transplantation
*** In addition to spinach leaves, the team successfully removed cells from parsley, Artemesia annua (sweet wormwood), and peanut hairy roots.
**** “Tissue engineered scaffolds are typically produced either from animal-derived or synthetic biomaterials, both of which have a large cost and large environmental impact. Animal-derived biomaterials used extensively as scaffold materials for tissue engineering include native [extracellular matrix] proteins such as collagen I or fibronectin and whole animal tissues and organs. Annually, 115 million animals are estimated to be used in research. Due to this large number, a lot of energy is necessary for the upkeep and feeding of such animals as well as to dispose of the large amount of waste that is generated. Along with this environmental impact, animal research also has a plethora of ethical considerations, which could be alleviated by forgoing animal models in favor of more biologically relevant in vitro human tissue models,” the authors advise.
Worcester Polytechnic Institute | Spinach leaves can carry blood to grow human tissues