Scientists map mammalian neural microcircuits in precise detail
January 12, 2018
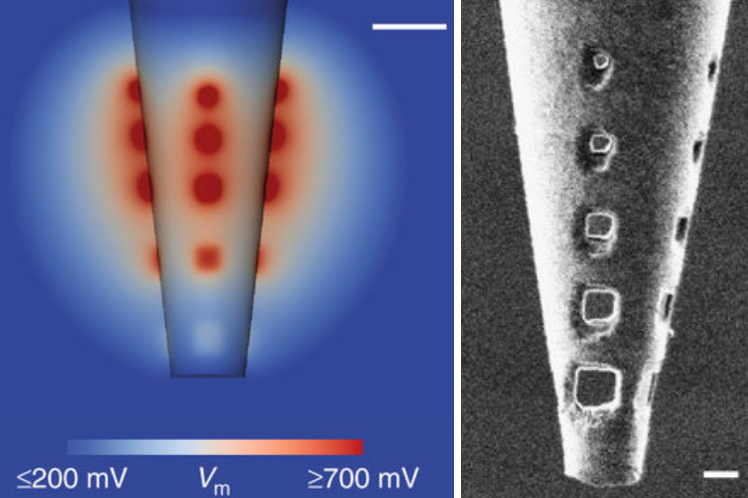
Nanoengineered electroporation microelectrodes (NEMs) allow for improved current distribution and electroporation effectiveness by reducing peak voltage regions (to avoid damaging tissue). (left) Cross-section of NEM model, illustrating the total effective electroporation volume and its distribution of the voltage around the pipette tip, at a safe current of 50 microamperes. (Scale bar = 5 micrometers.) (right) A five-hole NEM after successful insertion into brain tissue, imaged with high-resolution focused ion beam (FIB). (Scale bar = 2 micrometers) (credit: D. Schwartz et al./Nature Communications)
Neuroscientists at the Francis Crick Institute have developed a new technique to map electrical microcircuits* in the brain at far more detail than existing techniques*, which are limited to tiny sections of the brain (or remain confined to simpler model organisms, like zebrafish).
In the brain, groups of neurons that connect up in microcircuits help us process information about things we see, smell and taste. Knowing how many neurons and other types of cells make up these microcircuits would give scientists a deeper understanding of how the brain computes complex information.
Nanoengineered microelectrodes
The researchers developed a new design called “nanoengineered electroporation** microelectrodes” (NEMs). They were able to use an NEM to map out all 250 cells that make up a specific microcircuit in a part of a mouse brain that processes smell (known as the “olfactory bulb glomerulus”) in a horizontal slice of the olfactory bulb — something never before achieved.
To do that, the team created a series of tiny pores (holes) near the end of a micropipette using nano-engineering tools. The new design distributes the electrical current uniformly over a wider area (up to a radius of about 50 micrometers — the size of a typical neural microcircuit), with minimal cell damage.
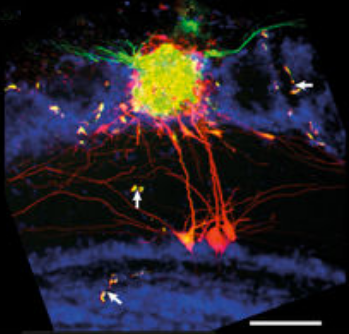
The researchers tested the NEM technique with a specific microcircuit, the olfactory bulb glomerulus (which detects smells). They were able to identify detailed, long-range, complex anatomical features (scale bar = 100 micrometers). (White arrows identify parallel staining of vascular structures.) (credit: D. Schwartz et al./Nature Communications)
Seeing 100% of the cells in a brain microcircuit for the first time
Unlike current methods, the team was able to stain up to 100% of the cells in the microcircuit they were investigating, according to Andreas Schaefer, who led the research, which was published in open-access Nature Communications today (Jan. 12, 2018).
“As the brain is made up of repeating units, we can learn a lot about how the brain works as a computational machine by studying it at this [microscopic] level,” he said. “Now that we have a tool of mapping these tiny units, we can start to interfere with specific cell types to see how they directly control behavior and sensory processing.”
The work was conducted in collaboration with researchers at the Max-Planck-Institute for Medical Research in Heidelberg, Heidelberg University, Heidelberg University Hospital, University College London, the MRC National Institute for Medical Research, and Columbia University Medical Center.
* Scientists currently use color-tagged viruses or charged dyes with applied electroporation current to stain brain cells. These methods, using a glass capillary with a single hole, are limited to low current (higher current could damage tissue), so they can only allow for identifying a limited area of a microcircuit.
** Electroporation is a microbiology technique that applies an electrical field to cells to increase the permeability (ease of penetration) of the cell membrane, allowing (in this case) fluorophores (fluorescent, or glowing dyes) to penetrate into the cells to label (identify parts of) the neural microcircuits (including the “inputs” and “outputs”) under a microscope.
Abstract of Architecture of a mammalian glomerular domain revealed by novel volume electroporation using nanoengineered microelectrodes
Dense microcircuit reconstruction techniques have begun to provide ultrafine insight into the architecture of small-scale networks. However, identifying the totality of cells belonging to such neuronal modules, the “inputs” and “outputs,” remains a major challenge. Here, we present the development of nanoengineered electroporation microelectrodes (NEMs) for comprehensive manipulation of a substantial volume of neuronal tissue. Combining finite element modeling and focused ion beam milling, NEMs permit substantially higher stimulation intensities compared to conventional glass capillaries, allowing for larger volumes configurable to the geometry of the target circuit. We apply NEMs to achieve near-complete labeling of the neuronal network associated with a genetically identified olfactory glomerulus. This allows us to detect sparse higher-order features of the wiring architecture that are inaccessible to statistical labeling approaches. Thus, NEM labeling provides crucial complementary information to dense circuit reconstruction techniques. Relying solely on targeting an electrode to the region of interest and passive biophysical properties largely common across cell types, this can easily be employed anywhere in the CNS.