Scientists reverse aging in mice by repairing damaged DNA
March 26, 2017
A research team led by Harvard Medical School professor of genetics David Sinclair, PhD, has made a discovery that could lead to a revolutionary new drug that allows cells to repair DNA damaged by aging, cancer, and radiation.
In a paper published in the journal Science on Friday (March 24), the scientists identified a critical step in the molecular process related to DNA damage.
The researchers found that a compound known as NAD (nicotinamide adenine dinucleotide), which is naturally present in every cell of our body, has a key role as a regulator in protein-to-protein interactions that control DNA repair. In an experiment, they found that treating mice with a NAD+ precursor called NMN (nicotinamide mononucleotide) improved their cells’ ability to repair DNA damage.
“The cells of the old mice were indistinguishable from the young mice, after just one week of treatment,” said senior author Sinclair.
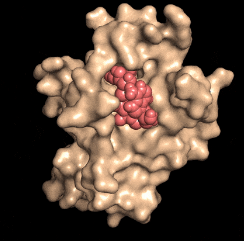
Disarming a rogue agent: When the NAD molecule (red) binds to the DBC1 protein (beige), it prevents DBC1 from attaching to and incapacitating a protein (PARP1) that is critical for DNA repair. (credit: David Sinclair)
Human trials of NMN therapy will begin within the next few months to “see if these results translate to people,” he said. A safe and effective anti-aging drug is “perhaps only three to five years away from being on the market if the trials go well.”
What it means for astronauts, childhood cancer survivors, and the rest of us
The researchers say that in addition to reversing aging, the DNA-repair research has attracted the attention of NASA. The treatment could help deal with radiation damage to astronauts in its Mars mission, which could cause muscle weakness, memory loss, and other symptoms (see “Mars-bound astronauts face brain damage from galactic cosmic ray exposure, says NASA-funded study“), and more seriously, leukemia cancer and weakened immune function (see “Travelers to Mars risk leukemia cancer, weakend immune function from radiation, NASA-funded study finds“).
The treatment could also help travelers aboard aircraft flying across the poles. A 2011 NASA study showed that passengers on polar flights receive about 12 percent of the annual radiation limit recommended by the International Committee on Radiological Protection.
The other group that could benefit from this work is survivors of childhood cancers, who are likely to suffer a chronic illness by age 45, leading to accelerated aging, including cardiovascular disease, Type 2 diabetes, Alzheimer’s disease, and cancers unrelated to the original cancer, the researchers noted.
For the past four years, Sinclair’s team has been working with spinoff MetroBiotech on developing NMN as a drug. Sinclair previously made a link between the anti-aging enzyme SIRT1 and resveratrol. “While resveratrol activates SIRT1 alone, NAD boosters [like NMN] activate all seven sirtuins, SIRT1-7, and should have an even greater impact on health and longevity,” he says.
Sinclair is also a professor at the University of New South Wales School of Medicine in Sydney, Australia.
Abstract of A conserved NAD+ binding pocket that regulates protein-protein interactions during aging
DNA repair is essential for life, yet its efficiency declines with age for reasons that are unclear. Numerous proteins possess Nudix homology domains (NHDs) that have no known function. We show that NHDs are NAD+ (oxidized form of nicotinamide adenine dinucleotide) binding domains that regulate protein-protein interactions. The binding of NAD+ to the NHD domain of DBC1 (deleted in breast cancer 1) prevents it from inhibiting PARP1 [poly(adenosine diphosphate–ribose) polymerase], a critical DNA repair protein. As mice age and NAD+ concentrations decline, DBC1 is increasingly bound to PARP1, causing DNA damage to accumulate, a process rapidly reversed by restoring the abundance of NAD+. Thus, NAD+ directly regulates protein-protein interactions, the modulation of which may protect against cancer, radiation, and aging.