Scientists transplant neural stem cells from a monkey’s skin into its brain
March 19, 2013
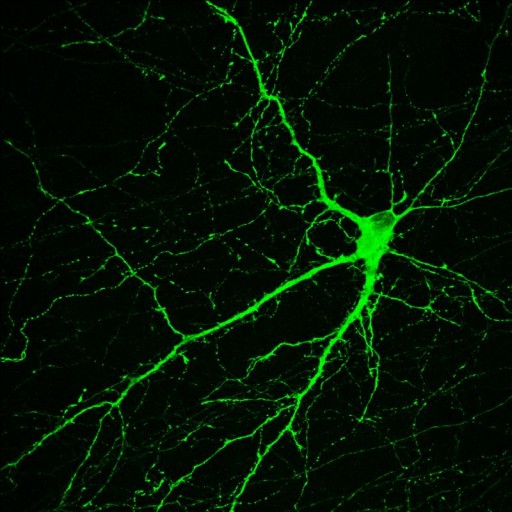
This monkey brain neuron, which originated from a skin stem cell, makes dopamine, a neurotransmitter involved in normal movement (credit: Yan Liu and Su-Chun Zhang)
University of Wisconsin-Madison scientists have transplanted neural cells derived from stem cells from a monkey’s skin into its brain and watched the cells develop into several types of mature brain cells.
After six months, the cells looked entirely normal, and were only detectable because they initially were tagged with a fluorescent protein.
The transplanted cells came from induced pluripotent stem cells (iPS cells), which can, like embryonic stem cells, develop into virtually any cell in the body. iPS cells, however, derive from adult cells rather than embryos.
In the lab, the iPS cells were converted into neural progenitor cells. These intermediate-stage cells can further specialize into the neurons that carry nerve signals, and the glial cells that perform many support and nutritional functions. This final stage of maturation occurred inside the monkey.
Since the skin cells were not “foreign” tissue, there were no signs of immune rejection — potentially a major problem with cell transplants.
“When you look at the brain, you cannot tell that it is a graft,” says senior author Su-Chun Zhang, a professor of neuroscience at the University of Wisconsin-Madison. “Structurally the host brain looks like a normal brain; the graft can only be seen under the fluorescent microscope.”
The cells were implanted in the monkeys using a surgical procedure guided by an MRI image. The three rhesus monkeys used in the study at the Wisconsin National Primate Research Center had a lesion in a brain region that causes the movement disorder Parkinson’s disease, which afflicts up to 1 million Americans. Parkinson’s is caused by the death of a small number of neurons that make dopamine, a signaling chemical used in the brain.
Another positive sign was the absence of any signs of cancer, says Zhang — a worrisome potential outcome of stem cell transplants. “Their appearance is normal, and we also used antibodies that mark cells that are dividing rapidly, as cancer cells are, and we do not see that. And [the cells] become neurons with long axons [conducting fibers], as we’d expect. They also produce oligodendrocytes, which help build insulating myelin sheaths for neuronsd. That means they have matured correctly, and are not cancerous.”
The experiment was designed as a proof of principle, says Zhang, who leads a group pioneering the use of iPS cells at the Waisman Center on the UW-Madison campus. The researchers did not transplant enough neurons to replace the dopamine-making cells in the brain, and the animal’s behavior did not improve.
“Unfortunately, this technique cannot be used to help patients until a number of questions are answered: Can this transplant improve the symptoms? Is it safe? Six months is not long enough… And what are the side effects? You may improve some symptoms, but if that leads to something else, then you have not solved the problem.”
“It’s really the first-ever transplant of iPS cells from a non-human primate back into the same animal,” says Zhang. “This proof-of-principle study in primates presents hope for personalized regenerative medicine.”