Scientists use stem cells to create human/pig chimera embryos
January 27, 2017
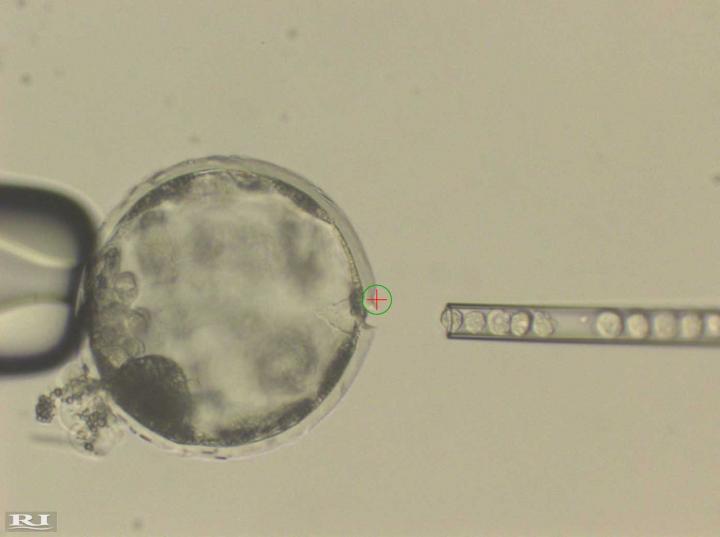
This photograph shows injection of human induced pluripotent stem* (iPS) cells into a pig blastocyst (pre-embryo). A laser beam (green circle with a red cross inside) was used to perforate an opening in the outer membrane (Zona Pellucida) of the pig blastocyst to allow easy access of an injection needle delivering human iPS cells. (credit: Courtesy of Juan Carlos Izpisua Belmonte)
In an open-access paper published online January 26, 2017 in the journal Cell, Salk Institute researchers report breakthroughs on multiple fronts in the race to integrate stem cells from one species into the early-stage development of another species (or chimeras**).
Scientists are still struggling to coax stem cells growing in Petri dishes to become fully functional specialized adult cells, the researchers report. “The ultimate goal is to grow functional and transplantable tissue or organs, but we are far away from that,” says lead investigator Juan Carlos Izpisua Belmonte, a professor in the Salk Institute of Biological Studies’ Gene Expression Laboratory. But their efforts to grow the first embryos containing cells from humans and pigs may someday provide a means of growing human cells, tissues, and organs for regenerative medicine. “This is an important first step.”
Meanwhile, the experiments are helping scientists understand how human stem cells grow and specialize, which may offer insights into disease onset or help establish new drug-testing platforms.
How to grow a chimera
In previous research, Izpisua Belmonte and Salk Institute staff scientist Jun Wu created a rat/mouse chimera by introducing rat cells into mouse embryos and letting the cells mature. The purpose was to find out whether rat-derived cells could rescue severe developmental defects in mouse pancreas, heart or lung. To do that, they used CRISPR genome editing tools to delete critical developmental genes in mouse egg cells. Remarkably, rat cells populated those mouse organs, filling in for the defective mouse cells.
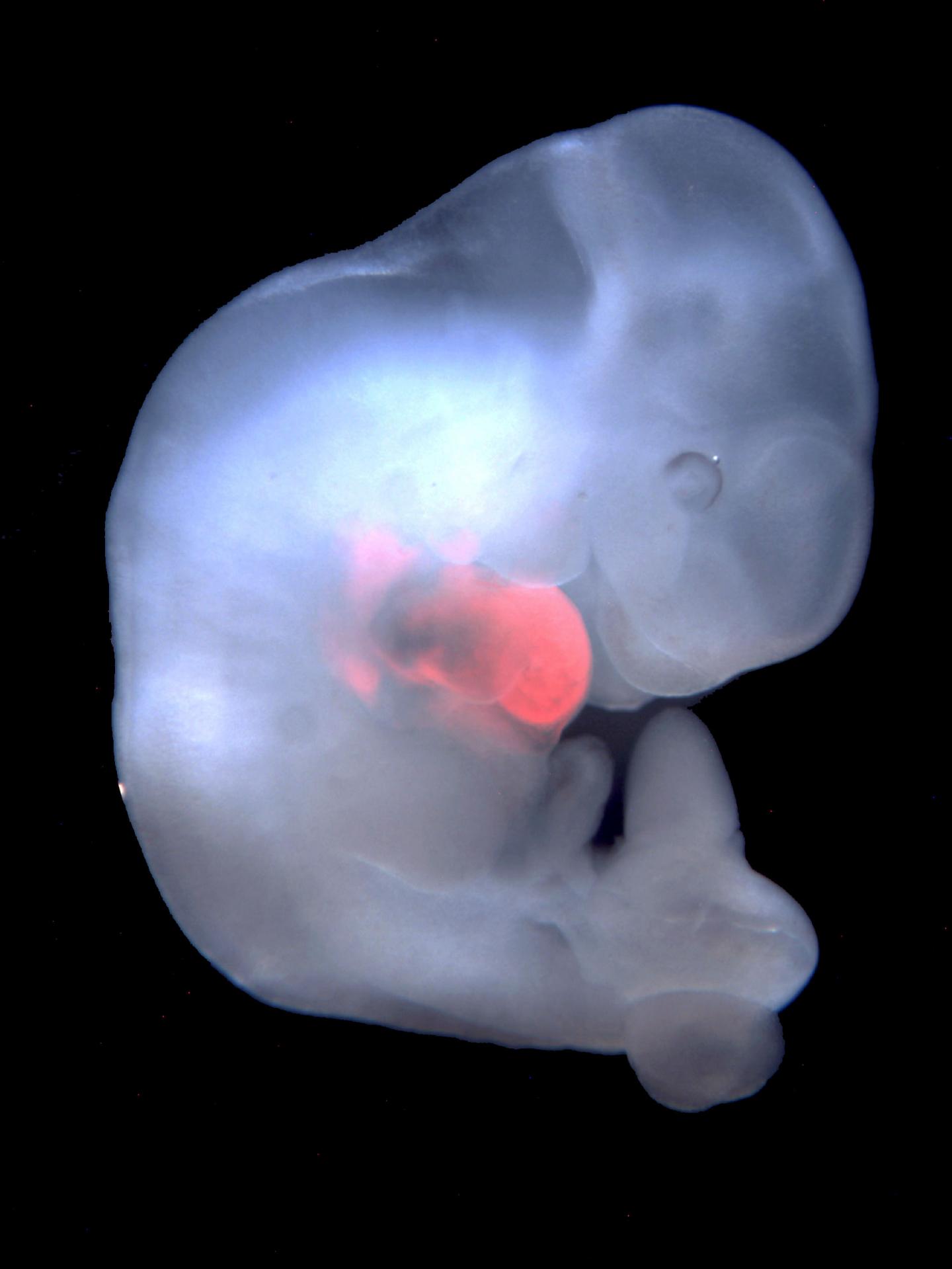
Here, cells derived from rat pluripotent stem cells were enriched in the developing heart of a genetically modified mouse embryo. (credit: Salk Institute)
For instance, in a given cell, the researchers would delete a single gene critical for the development of an organ, such as the heart, pancreas, or eye. Then they introduced rat stem cells into the embryos to see if they would fill the open niche. “The rat cells have a functional copy of the missing mouse gene, so they can outcompete mouse cells in occupying the emptied developmental organ niches,” says Wu. As the organism matured, the rat cells filled in where mouse cells could not, forming the functional tissues of the organism’s heart, eye, or pancreas.
Amazingly, in one experiment, rat cells also grew to form a gall bladder in the mouse, even though rats themselves stopped developing this organ over the 18 million years since rats and mice separated evolutionarily. “This suggests that the reason a rat does not generate a gall bladder is not because it cannot, but because the potential has been hidden by a rat-specific developmental program,” says Wu. “The microenvironment has evolved through millions of years to choose a program that defines a rat.”
Introducing human cells in pig embryos
The team’s next step was to introduce human cells into an organism. Experiments with cow embryos were difficult and costly. They decided to use pig embryos as hosts because the size of these animals’ organs more closely resembles those of humans than those of mice. The effort required completing studies with 1,500 pig embryos and involved the contributions of more than 40 people, including pig farmers, over a four-year period. “We underestimated the effort involved,” says Izpisua Belmonte. “This required a tour de force.”
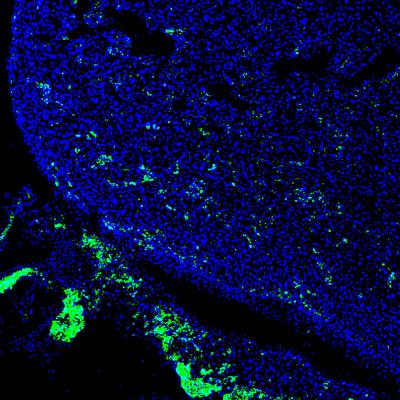
Salk scientists advance stem-cell and genome-editing technologies to help researchers study evolution and disease, test therapeutic drugs and possibly grow transplantable organs. Here, human iPS cells (green) contributed to a developing heart of 4-week-old pig embryo. (credit: Salk Institute)
The human cells survived and formed a human/pig chimera embryo. Embryos were implanted in sows and allowed to develop for between three and four weeks. “This is long enough for us to try to understand how the human and pig cells mix together early on without raising ethical concerns about mature chimeric animals,” says Izpisua Belmonte.
To ensure that, the human cells did not become precursors of brain cells that can grow into the central nervous system. Rather, they were developing into muscle cells and precursors of other organs. “At this point, we wanted to know whether human cells can contribute at all to address the ‘yes or no’ question,” he says. “Now that we know the answer is yes, our next challenge is to improve efficiency and guide the human cells into forming a particular organ in pigs.”
To do this, the researchers are using CRISPR to perform genome editing on the pig genome, as they did with mice, to open gaps that human cells can fill in. That work is in progress.
“Of course, the ultimate goal of chimeric research is to learn whether we can use stem-cell and gene-editing technologies to generate genetically matched human tissues and organs, and we are very optimistic that continued work will lead to eventual success,” says Izpisua Belmonte. “But in the process we are gaining a better understanding of species evolution as well as human embryogenesis and disease that is difficult to get in other ways.”
Other authors included scientists at the University of Murcia Campus de Espinardo, the University of California, Davis; the Universidad Católica San Antonio de Murcia; Clinica Centro Fundación Pedro Guillén; and the Hospital Clinic of Barcelona. The work was funded by The Fundación Séneca; a University of California, Davis, Academic Senate New Research Grant; the Universidad Católica San Antonio de Murcia; Fundacion Dr. Pedro Guillen; the G. Harold and Leila Y. Mathers Charitable Foundation; and The Moxie Foundation.
* Induced pluripotent stem cells (also known as iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from adult cells. They have the capacity to mature into many different types of cells and therefore can contribute to the formation of multiple organs or distinct cell lineages, but not all possible lineages. This developmental capacity differs from naïve stem cells, which can develop into any given cell type.
** An interspecies chimera is an organism containing cells from different species. The word “chimera” originally described mythological creatures or deities in polytheistic religions. In science, interspecies chimeras have emerged as valuable basic research tools with potential for future clinical applications.
Salk Institute | New findings highlight promise of chimeric organisms for science and medicine
Abstract of Interspecies Chimerism with Mammalian Pluripotent Stem Cells
Interspecies blastocyst complementation enables organ-specific enrichment of xenogenic pluripotent stem cell (PSC) derivatives. Here, we establish a versatile blastocyst complementation platform based on CRISPR-Cas9-mediated zygote genome editing and show enrichment of rat PSC-derivatives in several tissues of gene-edited organogenesis-disabled mice. Besides gaining insights into species evolution, embryogenesis, and human disease, interspecies blastocyst complementation might allow human organ generation in animals whose organ size, anatomy, and physiology are closer to humans. To date, however, whether human PSCs (hPSCs) can contribute to chimera formation in non-rodent species remains unknown. We systematically evaluate the chimeric competency of several types of hPSCs using a more diversified clade of mammals, the ungulates. We find that naïve hPSCs robustly engraft in both pig and cattle pre-implantation blastocysts but show limited contribution to post-implantation pig embryos. Instead, an intermediate hPSC type exhibits higher degree of chimerism and is able to generate differentiated progenies in post-implantation pig embryos.