Self-assembling smart scaffolds aim to rebuild tissue and future organs
November 14, 2012
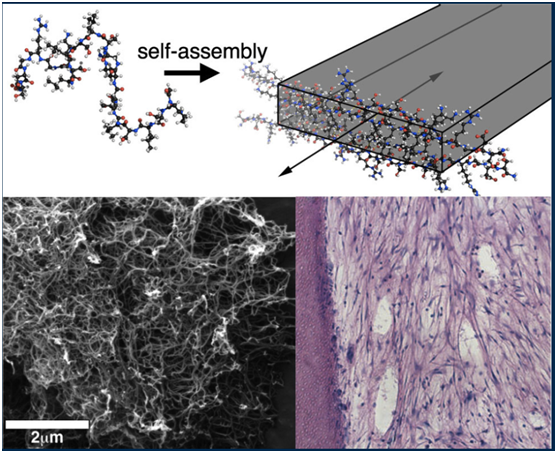
At top, a graphic shows multidomain peptide self-assembling into a nanofiber. The scanning electron microscope image at bottom left shows formed nanofibers; at bottom right, a histological section of cells (blue dots) grows in a dentin cylinder, where they mimic the desired dental-pulp regeneration. (Credit: Hartgerink Lab/Rice University)
A&M Health Science Center Baylor College of Dentistry has received a $1.7 million, five-year grant from the National Institutes of Health (NIH) to develop a hydrogel that can be injected into a patient to form an active biological scaffold for tooth repair, and possibly spinal cord regeneration, among other uses.
Rice bioengineer Jeffrey Hartgerink and co-investigator Rena D’Souza of Baylor won the grant to continue their groundbreaking work on self-assembling, multidomain peptide hydrogels, which physically support and encourage the growth of specific kinds of tissues.
Bioengineers use scaffolds to mimic the body’s extracellular matrix (ECM), which provides support for the growth and maintenance of living cells. These synthetic scaffolds are used as frameworks to form replacement tissues and, perhaps someday, regenerate entire organs from a patient’s own cells. The scaffolds are designed to degrade and leave only natural, healthy tissue behind.
While much of the work to date has focused on creating tissue in the laboratory for implantation, Hartgerink’s aim is to inject scaffolds infused with stem cells that will allow the repairs to happen inside the tissue’s natural environment.
A better solution for a toothache
The peptides (long molecular chains that make up proteins) self-assemble into nanofibers that can be triggered to form a hydrogel network. “We can then deliver cells, small-molecule drugs, and proteins to bring everything together properly in one place,” said Hartgerink, an associate professor of chemistry and of bioengineering at Rice. Hydrogels could be designed to interact with stem cells and “get them to do what we want them to do,” he said.
Hartgerink and D’Souza, a professor in the Department of Biomedical Sciences at Baylor currently on a working sabbatical at Rice’s BioScience Research Collaborative, have been pursuing the project for five years. The NIH grant will allow them to focus on the regeneration of the dentin-pulp complex found inside every tooth. The pulp, D’Souza said, is the soft tissue in the roots and crown that keeps the tooth vital and responsive to injury. “If you have a toothache, it’s the tissue that’s inflamed and has no place to expand. That’s why it hurts so much,” she said.
Currently, dentists remove inflamed pulp and replace it with an inert rubber-based filler, she said. But injecting stem cell-seeded hydrogels would allow natural pulp to regenerate into the chamber while stimulating new dentin formation. “Hydrogels have key advantages,” D’Souza said. “We can deliver them in a syringe to small spaces that are difficult to access, and the material does not get damaged. Developing this material as a restorative therapy is advantageous to patients as, unlike all other dental materials, this one is biologically active.”
The researchers reached a milestone in 2010 when they found a way to have the fibers degrade rather than stay in the body. With the new grant, they hope to start trials of their dental hydrogel within two years, D’Souza said. “I can see potential applications for hydrogel, for example, for spinal cord regeneration or for various eye conditions, where we can restore the vitreous humour,” she said.
The NIH grant is administered by the National Institute of Dental and Craniofacial Research.