Sorting out circulating tumor cells in the blood with sound waves
August 28, 2014
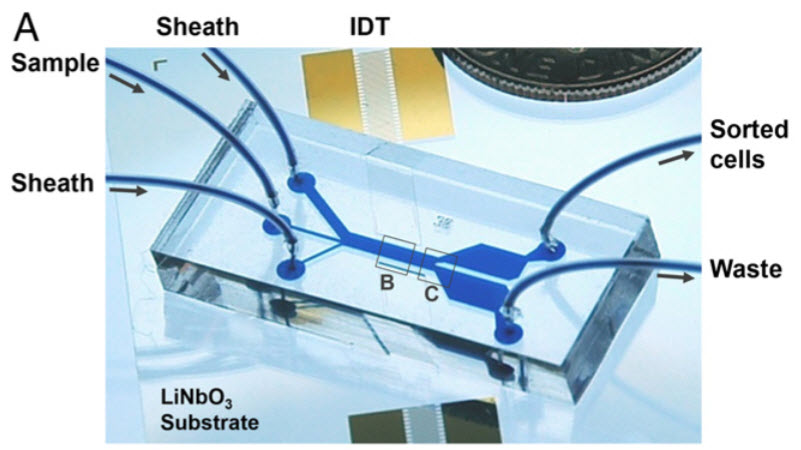
This microfluidic device uses sound waves to sort tumor from white-blood cells as they flow through the channel from left to right (IDT, or interdigital transducers, are sound source) (credit: X. Ding et al.)
A research team has developed a device that could be used to detect the extremely rare tumor cells that circulate in cancer patients’ blood, helping doctors predict whether a tumor is going to spread.
Developed by researchers from MIT, Pennsylvania State University, and Carnegie Mellon University, the dime-sized device separates out tumor cells from white blood cells by exposing the cells to sound waves as they flow through a tiny channel.
This is a gentler alternative to existing cell-sorting technologies, which require tagging the cells with chemicals or exposing them to stronger mechanical forces that may damage them.
“Acoustic pressure is very mild and much smaller in terms of forces and disturbance to the cell. This is a most gentle way to separate cells, and there’s no artificial labeling necessary,” says Ming Dao, a principal research scientist in MIT’s Department of Materials Science and Engineering and one of the senior authors of the paper, which appears this week in the Proceedings of the National Academy of Sciences (open access).
MIT | Researchers from MIT, Pennsylvania State University, and Carnegie Mellon
University have devised a new way to separate cells by exposing them to sound
waves as they flow through a tiny channel.
How it works
To sort cells using sound waves, scientists have previously built microfluidic devices with two acoustic transducers, which produce sound waves on either side of a microchannel. When the two waves meet, they combine to form a standing wave (a wave that remains in constant position). This wave produces a pressure node, or line of low pressure, running parallel to the direction of cell flow. Cells that encounter this node are pushed to the side of the channel; the distance of cell movement depends on their size and other properties such as compressibility.
However, because there is only one pressure node, cells can be pushed aside only short distances.
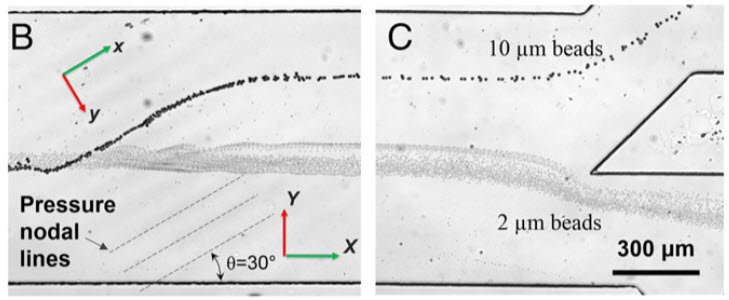
Separating larger cells (beads in this prototype) as they encounter the acoustic pressure nodes (credit: X. Ding et al.)
The new device overcomes that obstacle by tilting the sound waves (originating from IDT in figure A) so they run across the microchannel at an angle — each cell encounters several pressure nodes as it flows through the channel. Each time the cell encounters a node, the pressure guides the cell a little further off center, making it easier to capture cells of different sizes (figures B and C) by the time they reach the end of the channel.
This simple modification dramatically boosts the efficiency of such devices, says Taher Saif, a professor of mechanical science and engineering at the University of Illinois at Urbana-Champaign. “That is just enough to make cells of different sizes and properties separate from each other without causing any damage or harm to them,” says Saif, who was not involved in this work.
To test whether the device could be useful for detecting circulating tumor cells, the researchers tried to separate breast cancer cells known as MCF-7 cells from white blood cells. The device successfully recovered about 71 percent of the cancer cells; the researchers plan to test it with blood samples from cancer patients to see how well it can detect circulating tumor cells in clinical settings. Such cells are very rare: a 1-milliliter sample of blood may contain only a few tumor cells.
“This method is a step forward for detection of circulating tumor cells in the body. It has the potential to offer a safe and effective new tool for cancer researchers, clinicians and patients,” says Subra Suresh, president of Carnegie Mellon, the Vannevar Bush Professor of Engineering Emeritus, and a former dean of engineering at MIT.
The researchers have filed for a patent on the device. The research was funded by the National Institutes of Health and the National Science Foundation.
UPDATE 8/29/2014: In response to a KurzweilAI question, Xiaoyun Ding, an MIT postdoc and a lead author on the paper, said he believes the device could be enhanced, after further optimization and improvement, to “clean blood from some (if not all) types of circulating tumor cells. I am sure there is a chance.”
Another approach from Cornell University to deal with metastasizing cancer cells was reported on KurzweilAI in January: by injecting human blood samples, and later mice, with two proteins: E-selectin (ES, an adhesive) and TRAIL (Tumor Necrosis Factor Related Apoptosis-Inducing Ligand). The TRAIL protein when joined with the E-selectin protein was able to stick to leukocytes (white blood cells), which are abundant in the bloodstream. When a cancer cell comes into contact with TRAIL, which is nearly unavoidable in the frenzied flow of blood, the cancer cell essentially kills itself (apoptosis).
Abstract of Proceedings of the National Academy of Sciences paper
Separation of cells is a critical process for studying cell properties, disease diagnostics, and therapeutics. Cell sorting by acoustic waves offers a means to separate cells on the basis of their size and physical properties in a label-free, contactless, and biocompatible manner. The separation sensitivity and efficiency of currently available acoustic-based approaches, however, are limited, thereby restricting their widespread application in research and health diagnostics. In this work, we introduce a unique configuration of tilted-angle standing surface acoustic waves (taSSAW), which are oriented at an optimally designed inclination to the flow direction in the microfluidic channel. We demonstrate that this design significantly improves the efficiency and sensitivity of acoustic separation techniques. To optimize our device design, we carried out systematic simulations of cell trajectories, matching closely with experimental results. Using numerically optimized design of taSSAW, we successfully separated 2- and 10-µm-diameter polystyrene beads with a separation efficiency of ∼99%, and separated 7.3- and 9.9-µm-polystyrene beads with an efficiency of ∼97%. We illustrate that taSSAW is capable of effectively separating particles–cells of approximately the same size and density but different compressibility. Finally, we demonstrate the effectiveness of the present technique for biological–biomedical applications by sorting MCF-7 human breast cancer cells from nonmalignant leukocytes, while preserving the integrity of the separated cells. The method introduced here thus offers a unique route for separating circulating tumor cells, and for label-free cell separation with potential applications in biological research, disease diagnostics, and clinical practice.