Stress turns ordinary cells pluripotent [RESEARCHER MISCONDUCT FOUND]
January 30, 2014
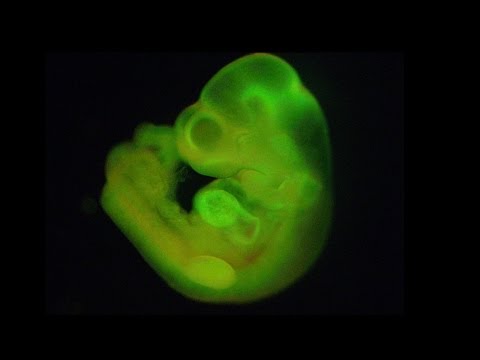
Chimeric mouse embryo generated from STAP cells (credit: RIKEN)
UPDATE: April 4, 2014 — A committee organized by the RIKEN Center for Developmental Biology has concluded that RIKEN’s Haruko Obokata, Ph.D., the lead researcher of this study, is guilty of scientific misconduct, according to a news article in Genetic Engineering & Biotechnology News.
————————————————–
Breakthrough findings by Haruko Obokata and colleagues at the RIKEN Center for Developmental Biology (CDB) look to upset the fundamental definitions of cellular differentiation and pluripotency.
The scientists were able to convert ordinary somatic cells from newborn mice to a state of pluripotency — meaning the converted cells could then differentiate (change) into any type of cell in the body. For example, a former muscle cell could become a neuron.
In many ways, the cells resembled that seen in embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).
The conversion process, which Obokata has named STAP (stimulus-triggered acquisition of pluripotency), requires only that the cells be shocked with a dose of sublethal stress, such as low pH or mechanical force, to trigger a remarkable transformation, in which the cells shrink, lose the functional characteristics specific to their somatic cell type, and enter a state of stem-cell-like pluripotency.
Such STAP cells show all the hallmarks of pluripotency, and contribute to chimeric mice and germline transmission when injected into early stage embryos.
Implications for regenerative medicine, aging, cancer
Even more interestingly, STAP cells show a level of plasticity that exceeds that even of ESCs and iPSCs, in that they can give rise to cells of both embryonic and extraembryonic (supporting cells outside the embryo itself) lineages; other pluripotent stem cells typically only generate embryonic lineage cells.
STAP cells also differ from stem cells in their lower ability to proliferate in culture, but Obokata found that by adding different factors to STAP culture medium, she was able to cause them to transform into either “STAP stem cells” (which behaved very much like embryonic stem cells), or a second form of stem cell capable of both generating extra-embryonic lineages and long-term culture.
“It’s exciting to think about the new possibilities these findings open up, not only in areas like regenerative medicine, but perhaps in the study of cellular senescence and cancer as well,” says Obokata. “But the greatest challenge for me going forward will be to dig deeper into the underlying mechanisms, so that we can gain a deeper understanding of how differentiated cells can covert to such an extraordinarily pluripotent state.”
This work was done in collaboration with Charles Vacanti’s lab at Brigham and Women’s Hospital, Harvard University, Masayuki Yamato’s lab at Tokyo Women’s Medical University, and the laboratories for Genomic Reprogramming, Pluripotent Stem Cell Studies, and Organogenesis and Neurogenesis at the RIKEN CDB.
Abstract of Nature paper: Stimulus-triggered fate conversion of somatic cells into pluripotency
Here we report a unique cellular reprogramming phenomenon, called stimulus-triggered acquisition of pluripotency (STAP), which requires neither nuclear transfer nor the introduction of transcription factors. In STAP, strong external stimuli such as a transient low-pH stressor reprogrammed mammalian somatic cells, resulting in the generation of pluripotent cells. Through real-time imaging of STAP cells derived from purified lymphocytes, as well as gene rearrangement analysis, we found that committed somatic cells give rise to STAP cells by reprogramming rather than selection. STAP cells showed a substantial decrease in DNA methylation in the regulatory regions of pluripotency marker genes. Blastocyst injection showed that STAP cells efficiently contribute to chimaeric embryos and to offspring via germline transmission. We also demonstrate the derivation of robustly expandable pluripotent cell lines from STAP cells. Thus, our findings indicate that epigenetic fate determination of mammalian cells can be markedly converted in a context-dependent manner by strong environmental cues.
Abstract of Nature paper: Bidirectional developmental potential in reprogrammed cells with acquired pluripotency
We recently discovered an unexpected phenomenon of somatic cell reprogramming into pluripotent cells by exposure to sublethal stimuli, which we call stimulus-triggered acquisition of pluripotency (STAP). This reprogramming does not require nuclear transferor genetic manipulation. Here we report that reprogrammed STAP cells, unlike embryonic stem (ES) cells, can contribute to both embryonic and placental tissues, as seen in a blastocyst injection assay. Mouse STAP cells lose the ability to contribute to the placenta as well as trophoblast marker expression on converting into ES-like stem cells by treatment with adrenocorticotropic hormone (ACTH) and leukaemia inhibitory factor (LIF). In contrast, when cultured with Fgf4, STAP cells give rise to proliferative stem cells with enhanced trophoblastic characteristics. Notably, unlike conventional trophoblast stem cells, the Fgf4-induced stem cells from STAP cells contribute to both embryonic and placental tissues in vivo and transform into ES-like cells when cultured with LIF-containing medium. Taken together, the developmental potential of STAP cells, shown by chimaera formation and in vitro cell conversion, indicates that they represent a unique state of pluripotency.