The geometry of immune system cloaking
May 19, 2015
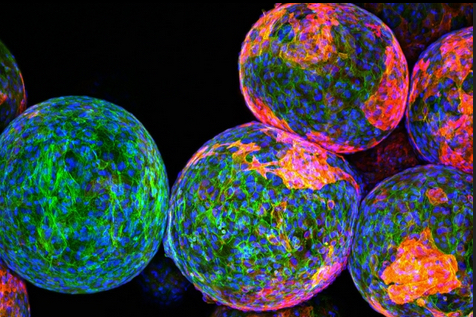
Cloaking materials: The sugar polymers that make up the spheres in this image are designed to package and protect specially engineered cells that work to produce drugs and fight disease and remain undetected by the body’s natural defense system. However, the reddish markers on the spheres’ surfaces indicate that immune cells (blue/green) have discovered these invaders and begun to block them off from the rest of the body. — credit | MIT researchers
A team of MIT researchers has come up with a way to reduce immune-system rejection of implantable devices used for for drug delivery, tissue engineering, or sensing.
Previous research found that smooth surfaces, especially spheres, are better — but counterintuively, larger spheres actually work better at reducing scar tissue, the researchers discovered.
“We were surprised by how much the size and shape of an implant can affect its triggering of an immune response. What it’s made of is still an important piece of the puzzle, but it turns out if you really want to have the least amount of scar tissue you need to pick the right size and shape,” says Daniel Anderson, the Samuel A. Goldblith Associate Professor in MIT’s Department of Chemical Engineering, a member of MIT’s Koch Institute for Integrative Cancer Research and Institute for Medical Engineering and Science (IMES), and the paper’s senior author.
Tests of spheres
The study grew out of the researchers’ efforts to build an artificial pancreas. The goal is to deliver pancreatic islet cells encapsulated within a particle made of alginate — a polysaccharide (sugar) naturally found in algae — or another material. These implanted cells could replace patients’ pancreatic islet cells, which are nonfunctional in Type I diabetes.
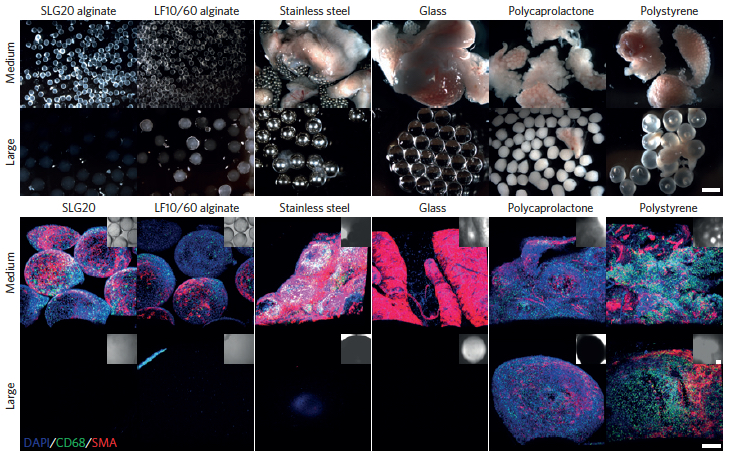
Increasing the spherical diameter of a variety of materials including hydrogels, ceramics, metals and plastics (a) — scale bar: 2 millimeters — results in reduced foreign-body responses (b) — scale bar: 300 micrometers (credit: Omid Veiseh et al./Nature Materials)
The researchers tested spheres in two sizes — 0.5 and 1.5 millimeters in diameter. In tests of diabetic mice, the spheres were implanted within the abdominal cavity and the researchers tracked their ability to accurately respond to changes in glucose levels. The devices prepared with the smaller spheres were completely surrounded by scar tissue and failed after about a month, while the larger ones were not rejected and continued to function for more than six months.
The larger spheres also evaded the immune response in tests in nonhuman primates. Smaller spheres implanted under the skin were engulfed by scar tissue after only two weeks, while the larger ones remained clear for up to four weeks.
A universal size effect
This effect was seen not only with alginate, but also with spheres made of stainless steel, glass, polystyrene, and polycaprolactone, a type of polyester. “We realized that regardless of what the composition of the material is, this effect still persists, and that made it a lot more exciting because it’s a lot more generalizable,” said Koch Institute postdoc Omid Veiseh, one of the lead authors of a paper in the May 18 issue of Nature Materials.
The researchers believe this finding could also be applicable to any other type of implantable device, including drug-delivery vehicles and sensors for glucose and insulin, which could also help improve diabetes treatment. Optimizing particle size and shape could also help guide scientists in developing other types of implantable cells for treating diseases other than diabetes.
The research was funded by the Juvenile Diabetes Research Foundation, the Leona M. and Harry B. Helmsley Charitable Trust Foundation, the National Institutes of Health, the Koch Institute Support Grant from the National Cancer Institute, and the Tayebati Family Foundation. Veiseh was also supported by the Department of Defense.
Abstract of Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates
The efficacy of implanted biomedical devices is often compromised by host recognition and subsequent foreign body responses. Here, we demonstrate the role of the geometry of implanted materials on their biocompatibility in vivo. In rodent and non-human primate animal models, implanted spheres 1.5 mm and above in diameter across a broad spectrum of materials, including hydrogels, ceramics, metals and plastics, significantly abrogated foreign body reactions and fibrosis when compared with smaller spheres. We also show that for encapsulated rat pancreatic islet cells transplanted into streptozotocin-treated diabetic C57BL/6 mice, islets prepared in 1.5-mm alginate capsules were able to restore blood-glucose control for up to 180 days, a period more than five times longer than for transplanted grafts encapsulated within conventionally sized 0.5-mm alginate capsules. Our findings suggest that the in vivo biocompatibility of biomedical devices can be significantly improved simply by tuning their spherical dimensions.