The heat is on for sodium-manganese oxide rechargeable batteries
June 8, 2011
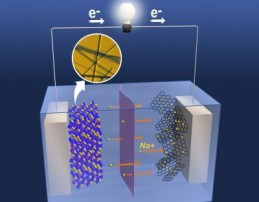
The uniform nanostructure of heat-treated manganese oxide provides tunnels for sodium ions to flow through, improving the performance of the electrodes (credit: PNNL)
Researchers at the DOE’s Pacific Northwest National Laboratory and visiting researchers from Wuhan University, China, have developed a method that improves the electrical capacity and recharging lifetime of sodium ion rechargeable batteries.
It’s a potentially cheaper alternative for large-scale uses, such as storing energy on the electrical grid.
The team mixed two different kinds of manganese oxide atomic building blocks: one whose atoms arrange themselves in pyramids, and another one whose atoms form an octahedron. They expected the final material to have large S-shaped tunnels and smaller five-sided tunnels through which the ions could flow.
After mixing, the team treated the materials with temperatures ranging from 450 to 900 degrees Celsius, then examined the materials and tested which treatment worked best. Using a scanning electron microscope, the researchers found that different temperatures created material of different quality. Treating the manganese oxide at 750 degrees Celsius created the best crystals: too low and the crystals appeared flakey, too high and the crystals turned into larger flat plates.
The team measured peak capacity at 128 milliamp-hours per gram of electrode material as an experimental battery cell discharged.
The material also held up well to cycles of charging and discharging. The material treated at 750 Celsius performed the best: after 100 cycles of charging-discharging, it lost only 7 percent of its capacity.
Ref.: Zhenguo Yang and Jun Liu, et al., Reversible Sodium Ion Insertion in Single Crystalline Manganese Oxide Nanowires with Long Cycle Life, Advanced Materials, June 3, 2011, [DOI 10.1002/adma.201100904 ]
Also see: New battery design could be breakthrough for electric vehicles and grid storage