Tiny electronic implants monitor brain injury, then melt away
January 19, 2016
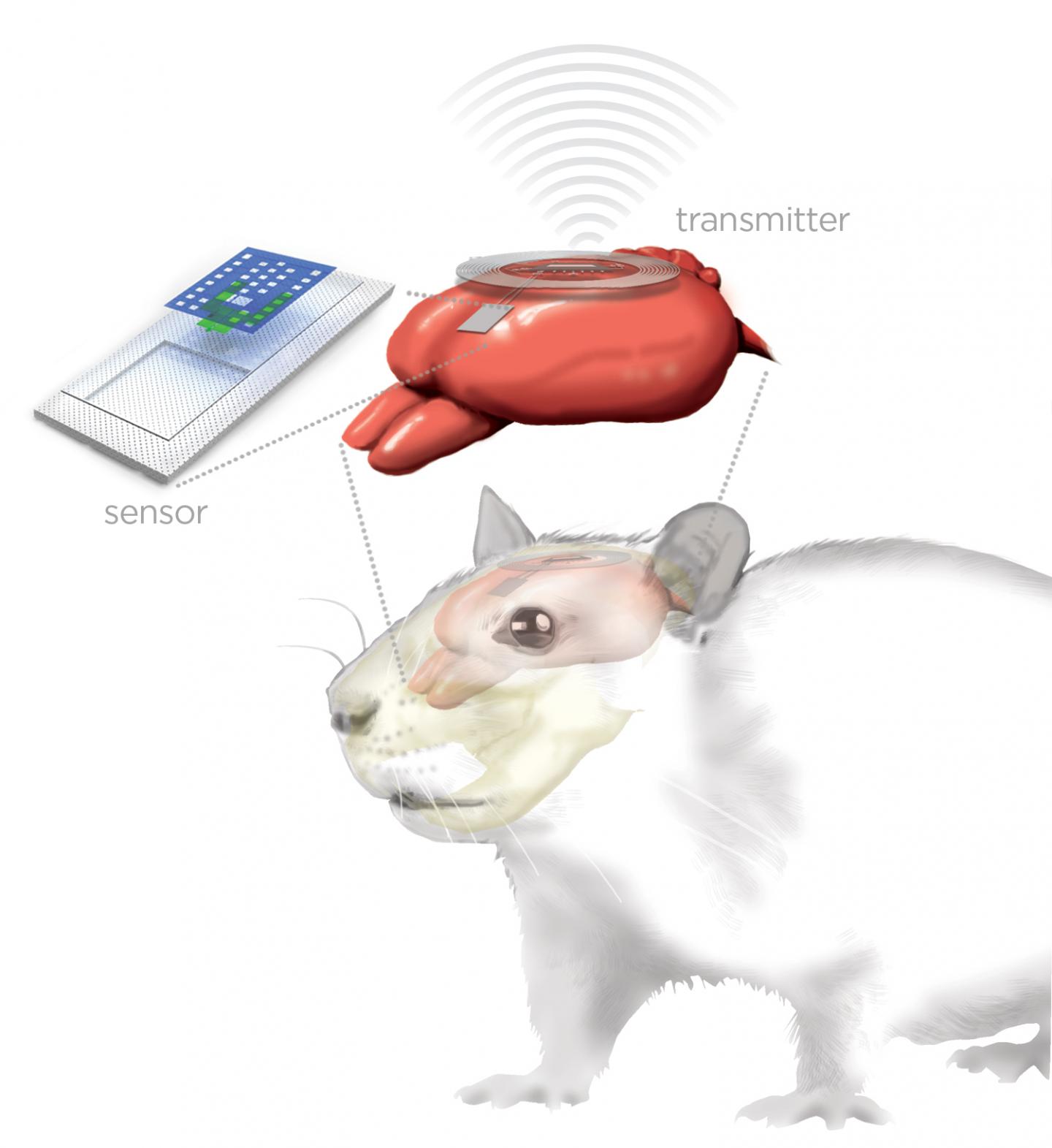
Artist’s rendering of bioresorbable implanted brain sensor (top left) connected via biodegradable wires to external wireless transmitter (ring, top right) for monitoring a rat’s brain (red) (credit: Graphic by Julie McMahon)
Researchers at University of Illinois at Urbana-Champaign and Washington University School of Medicine in St. Louis have developed a new class of small, thin electronic sensors that can monitor temperature and pressure within the skull — crucial health parameters after a brain injury or surgery* — then melt away when they are no longer needed, eliminating the need for additional surgery to remove the monitors and reducing the risk of infection and hemorrhage.
Similar sensors could be adapted for postoperative monitoring in other body systems as well, the researchers say.
John A. Rogers, a professor of materials science and engineering at the at the U. of I. at Urbana-Champaign, and Wilson Ray, a professor of neurological surgery at Washington University, published their work in the journal Nature.
After a traumatic brain injury or brain surgery, it’s crucial to monitor the patient for swelling and pressure on the brain. Current monitoring technology is bulky and invasive, Rogers said, and the wires restrict the patent’s movement and hamper physical therapy as they recover. Because they require continuous, hard-wired access into the head, such implants also carry the risk of allergic reactions, infection and hemorrhage, and even could exacerbate the inflammation they are meant to monitor.
Bioresorbable materials
“If you simply could throw out all the conventional hardware and replace it with very tiny, fully implantable sensors capable of the same function, constructed out of bioresorbable materials in a way that also eliminates or greatly miniaturizes the wires, then you could remove a lot of the risk and achieve better patient outcomes,” Rogers said. ”We were able to demonstrate all of these key features in animal models, with a measurement precision that’s just as good as that of conventional devices.”
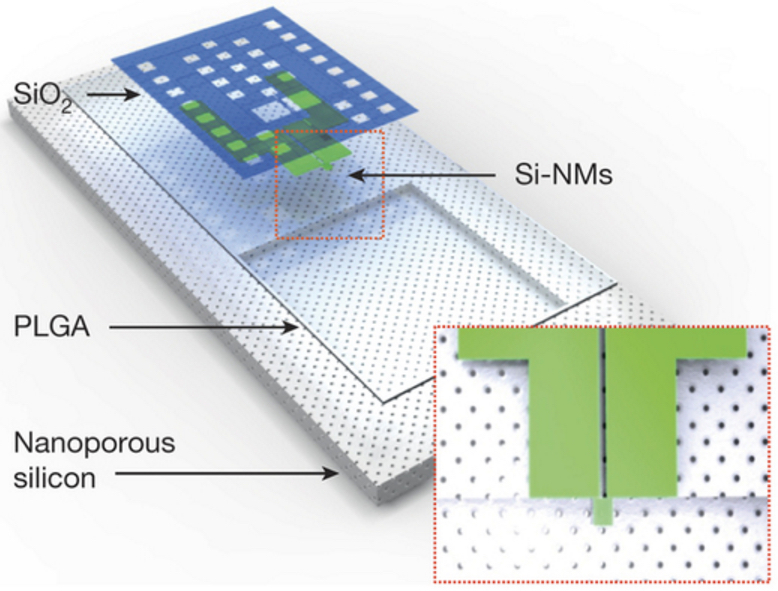
Schematic illustration of a biodegradable pressure sensor. The inset shows the location of the silicon-nanomembrane (Si-NM) strain gauge. (credit: Seung-Kyun Kang et al./Nature)
The new devices incorporate dissolvable silicon technology developed by Rogers’ group at the U. of I. The sensors, smaller than a grain of rice, are built on extremely thin sheets of nanoporous silicon — which are naturally biodegradable. The sheets are configured to function normally for a few weeks, then dissolve away, completely and harmlessly, in the body’s own fluids (via hydrolysis and/or metabolic action).
The silicon platforms are also sensitive to clinically relevant pressure levels in the intracranial fluid surrounding the brain.
The researchers added a tiny temperature sensor and connected it to a wireless transmitter roughly the size of a postage stamp, implanted under the skin but on top of the skull.
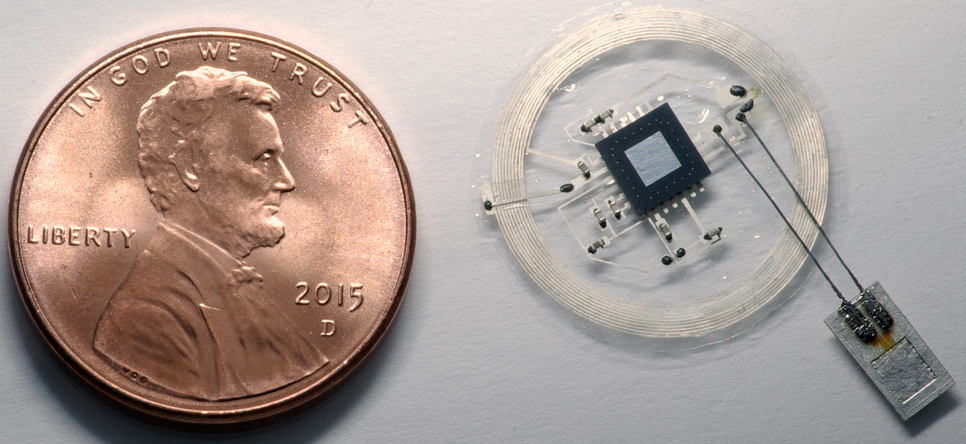
Tiny pressure and temperature sensor (bottom right) connects via bioresorbable molybdenum wires (2-micrometers in diameter) to a penny-size wireless transmitter externally mounted on top of the skull. (image credit: John A. Rogers)
The Illinois group worked with clinical experts in traumatic brain injury at Washington University to implant the sensors in rats, testing for performance and biocompatibility. They found that the temperature and pressure readings from the dissolvable sensors matched conventional monitoring devices for accuracy.
“The ultimate strategy is to have a device that you can place in the brain — or in other organs in the body — that is entirely implanted, intimately connected with the organ you want to monitor and can transmit signals wirelessly to provide information on the health of that organ, allowing doctors to intervene if necessary to prevent bigger problems,” said Rory Murphy, a neurosurgeon at Washington University and co-author of the paper.
“After the critical period that you actually want to monitor, it will dissolve away and disappear.”
Embedding drug-delivery and electrical-stimulator devices
The researchers are moving toward human trials for this technology, as well as extending its functionality for other biomedical applications.
“We have established a range of device variations, materials, and measurement capabilities for sensing in other clinical contexts,” Rogers said. “In the near future, we believe that it will be possible to embed therapeutic function, such as electrical stimulation or drug delivery, into the same systems while retaining the essential bioresorbable character.”
The National Institutes of Health, the Defense Advanced Research Projects Agency and the Howard Hughes Medical Institute supported this work. Rogers and Braun are affiliated with the Beckman Institute for Advanced Science and Technology at the U. of I.
* About 50,000 people die of traumatic brain injuries annually in the U.S. When patients with such injuries arrive in the hospital, doctors must be able to accurately measure intracranial pressure in the brain and inside the skull because an increase in pressure can lead to further brain injury, and there is no way to reliably estimate pressure levels from brain scans or clinical features in patients.
Abstract of Bioresorbable silicon electronic sensors for the brain
Many procedures in modern clinical medicine rely on the use of electronic implants in treating conditions that range from acute coronary events to traumatic injury. However, standard permanent electronic hardware acts as a nidus for infection: bacteria form biofilms along percutaneous wires, or seed haematogenously, with the potential to migrate within the body and to provoke immune-mediated pathological tissue reactions. The associated surgical retrieval procedures, meanwhile, subject patients to the distress associated with re-operation and expose them to additional complications. Here, we report materials, device architectures, integration strategies, and in vivo demonstrations in rats of implantable, multifunctional silicon sensors for the brain, for which all of the constituent materials naturally resorb via hydrolysis and/or metabolic action, eliminating the need for extraction. Continuous monitoring of intracranial pressure and temperature illustrates functionality essential to the treatment of traumatic brain injury; the measurement performance of our resorbable devices compares favourably with that of non-resorbable clinical standards. In our experiments, insulated percutaneous wires connect to an externally mounted, miniaturized wireless potentiostat for data transmission. In a separate set-up, we connect a sensor to an implanted (but only partially resorbable) data-communication system, proving the principle that there is no need for any percutaneous wiring. The devices can be adapted to sense fluid flow, motion, pH or thermal characteristics, in formats that are compatible with the body’s abdomen and extremities, as well as the deep brain, suggesting that the sensors might meet many needs in clinical medicine.