Tunable brain cells that morph on demand
September 16, 2015
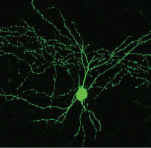
PV+ interneuron (credit: Nathalie Dehorter et al./Science)
King’s College London researchers have developed a new molecular “switch” that controls the properties of certain neurons in response to changes in the activity of their neural network — suggesting that these circuits in our brain are tuneable and could have implications that go far beyond basic neuroscience.
The researchers, from the MRC Centre for Developmental Neurobiology (MRC CDN) at the Institute of Psychiatry, Psychology & Neuroscience (IoPPN), led by Professor Oscar Marín, have discovered that some neurons in the cerebral cortex can adapt their properties in response to changes in network activity, such as learning a motor (muscle) task.
The authors studied two apparently different classes of fast-spiking interneurons but discovered they were actually looking at the same neuron — one with the ability to oscillate between two different ground states. The authors then identified the molecular factor responsible for tuning the properties of these cells: a transcription factor (a protein able to influence gene expression) known as Er81.
Neurons with “tremendous plasticity”
Fast-spiking interneurons, known as FS PV+, are members of a general class of neurons whose primary role is regulating the activity of pyramidal cells (the principal cells of the cerebral cortex). These PV+ interneurons play a prominent role in the regulation of plasticity and learning.
The researchers believe that PV+ interneurons take on properties based on how they adapt and respond to internal and external influences to encode information. “In other words, that our [brain’s] ‘hardware’ is tuneable, at least to some extent,” said Nathalie Dehorter of the MRC CDN and first author of the study, published in the journal Science on Sept. 11, 2015.
“Our study demonstrates the tremendous plasticity of the brain, and how this relates to fundamental processes such as learning,” said Marín. “Understanding the mechanisms that regulate this plasticity, and why it tends to dissipate when we age, has enormous implications that go beyond fundamental neuroscience, from informing education policies to developing new therapies for neurological disorders such as epilepsy.”
Abstract of Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch
The function of neural circuits depends on the generation of specific classes of neurons. Neural identity is typically established near the time when neurons exit the cell cycle to become postmitotic cells, and it is generally accepted that, once the identity of a neuron has been established, its fate is maintained throughout life. Here, we show that network activity dynamically modulates the properties of fast-spiking (FS) interneurons through the postmitotic expression of the transcriptional regulator Er81. In the adult cortex, Er81 protein levels define a spectrum of FS basket cells with different properties, whose relative proportions are, however, continuously adjusted in response to neuronal activity. Our findings therefore suggest that interneuron properties are malleable in the adult cortex, at least to a certain extent.