Turning human stem cells into brain cells sheds light on neural development
May 6, 2013
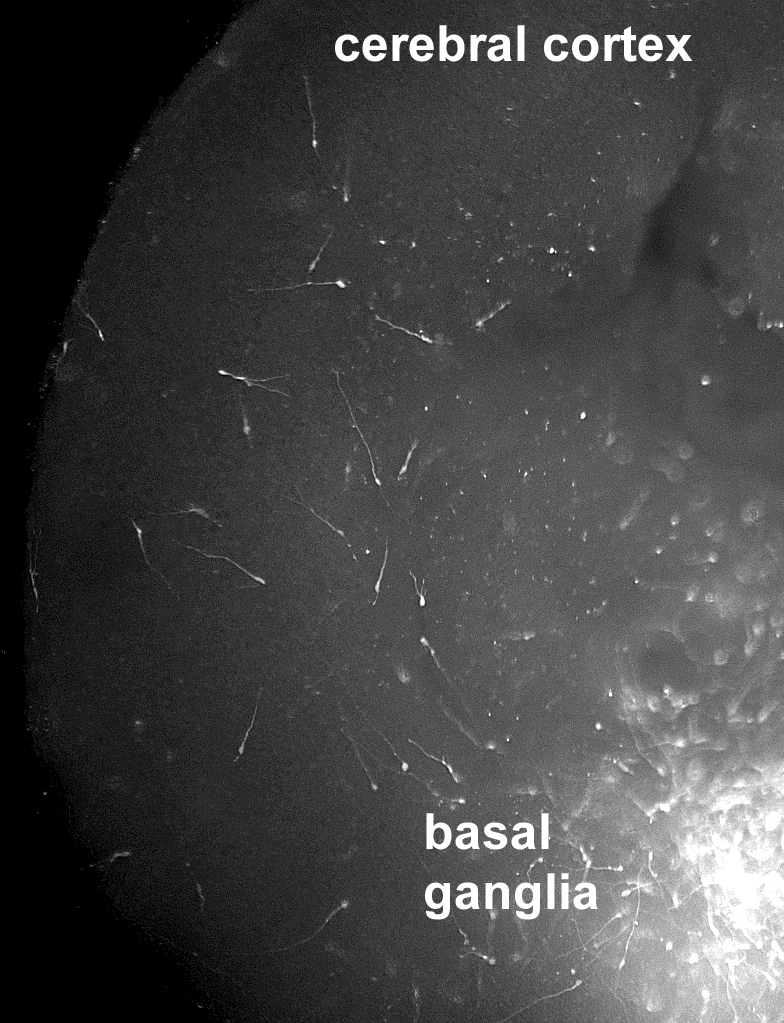
Human stem cell derived cortical interneuron precursors, placed into the basal ganglia region of an embryonic mouse brain slice, can follow evolutionary conserved migratory cues that guide them to migrate into the overlying cerebral cortex. This system can serve as a platform for studying disorders in which interneuron migration abnormalities are either known to occur, as in some cases of infantile epilepsy, or have been postulated to occur, as in some cases of autism and schizophrenia. (Credit: Children’s Hospital of Philadelphia
Scientists from The Children’s Hospital of Philadelphia and the Sloan-Kettering Institute for Cancer Research have led a study team that has manipulated human stem cells into producing types of brain cells known to play important roles in neurodevelopmental disorders such as epilepsy, schizophrenia and autism.
The new model cell system allows neuroscientists to investigate normal brain development, as well as to identify specific disruptions in biological signals that may contribute to neuropsychiatric diseases.
The research harnesses human embryonic stem cells (hESCs), which differentiate into a broad range of different cell types. In the current study, the scientists directed the stem cells into becoming cortical interneurons — a class of brain cells that, by releasing the neurotransmitter GABA, controls electrical firing in brain circuits.
“Interneurons act like an orchestra conductor, directing other excitatory brain cells to fire in synchrony,” said study co-leader Stewart A. Anderson, M.D., a research psychiatrist at The Children’s Hospital of Philadelphia. “However, when interneurons malfunction, the synchrony is disrupted, and seizures or mental disorders can result.”
Anderson and study co-leader Lorenz Studer, M.D., of the Center for Stem Cell Biology at Sloan-Kettering, derived interneurons in a laboratory model that simulates how neurons normally develop in the human forebrain.
The human-derived cells in the current study “wire up” in circuits with other types of brain cells taken from mice, when cultured together. Those interactions, Anderson added, allowed the study team to observe cell-to-cell signaling that occurs during forebrain development.
In ongoing studies, Anderson explained, he and colleagues are using their cell model to better define molecular events that occur during brain development. By selectively manipulating genes in the interneurons, the researchers seek to better understand how gene abnormalities may disrupt brain circuitry and give rise to particular diseases. Ultimately, those studies could help inform drug development by identifying molecules that could offer therapeutic targets for more effective treatments of neuropsychiatric diseases.
In addition, Anderson’s laboratory is studying interneurons derived from stem cells made from skin samples of patients with chromosome 22q.11.2 deletion syndrome, a genetic disease which has long been studied at The Children’s Hospital of Philadelphia.
In this multisystem disorder, about one third of patients have autistic spectrum disorders, and a partially overlapping third of patients develop schizophrenia. Investigating the roles of genes and signaling pathways in their model cells may reveal specific genes that are crucial in those patients with this syndrome who have neurodevelopmental problems.
Grants from the National Institute of Mental Health (MH066912, MH089690), NYSTEM, NeuroStemcell, the C.V. Starr Foundation and the Swiss National Science Foundation supported parts of this study.