Two-dimensional ‘electron gas’ creates radical microelectronics devices
March 4, 2014
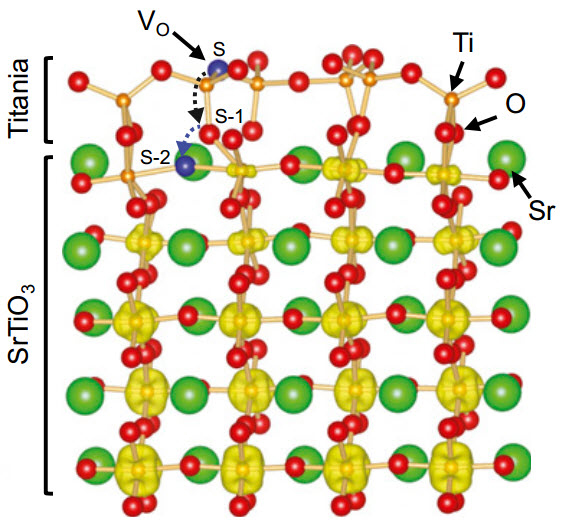
Atomic structure of strontium titinate. An oxygen vacancy
(Vo) formed at the reconstructed surface spontaneously migrates to the plane beneath the top titania layer. The excess electrons from the Vo form a two-dimensional electron gas confined within a region of about 2 nm thickness. (Credit: Vienna University of Technology)
Vienna University of Technology researchers have created a two-dimensional “electron gas” in strontium titanate. In a thin layer formed by the gas just below the surface, electrons can move freely and occupy different quantum states.
The new material represents a potential future alternative to standard semiconductors. Strontium titanate or other metal oxides could also exhibit other novel phenomena, such as superconductivity, thermoelectricity (converting heat to electricity), or magnetic effects, which cannot be achieved with materials used for today’s chips.
Creating an ‘electron gas’
“Inside, every titanium atom has six neighboring oxygen atoms, whereas the titanium atoms at the surface are only connected to four oxygen atoms each”, says professor Ulrike Diebold.
“This is the reason for the remarkable chemical stability of the surface. Normally such materials are damaged if they come into contact with water or oxygen.”
Something remarkable happens when the material is irradiated with high-energy electromagnetic waves. “The radiation can remove oxygen atoms from the surface,” Diebold explains. Then other oxygen atoms from within the bulk of the material move up to the surface. Inside the material, an oxygen deficiency builds up, as well a surplus of electrons.
“These electrons, located in a two dimensional layer very close to the surface, can then move freely. We call this an electron gas,” says professor Karsten Held. There has already been some evidence of two-dimensional electron gases in similar materials, but until now, the creation of a stable, durable electron gas at a surface has been impossible.
The properties of the electrons in the gas can also be finely tuned. Depending on the intensity of the radiation, the number of electrons varies. By adding different atoms, the band structure, and thus the electronic properties, can also be changed.
The electron gas in the new material exhibits a multitude of different electronic structures. Some of them could very well be suitable for producing interesting magnetic effects or superconductivity. The researchers hope that, by applying external electric fields or by placing additional metal atoms on the surface, the new material could reveal a few more of its secrets.
Abstract of Proceedings of the National Academy of Sciences paper
Two-dimensional electron gases (2DEGs) at oxide heterostructures are attracting considerable attention, as these might one day substitute conventional semiconductors at least for some functionalities. Here we present a minimal setup for such a 2DEG – the SrTiO3(110)-(4 × 1) surface, natively terminated with one monolayer of tetrahedrally coordinated titania. Oxygen vacancies induced by synchrotron radiation migrate underneath this overlayer; this leads to a confining potential and electron doping such that a 2DEG develops. Our angle-resolved photoemission spectroscopy and theoretical results show that confinement along (110) is strikingly different from the (001) crystal orientation. In particular, the quantized subbands show a surprising “semiheavy” band, in contrast with the analog in the bulk, and a high electronic anisotropy. This anisotropy and even the effective mass of the (110) 2DEG is tunable by doping, offering a high flexibility to engineer the properties of this system.