Ultra-high-resolution movies of live 3D biomolecules now possible with new microscope
October 23, 2014
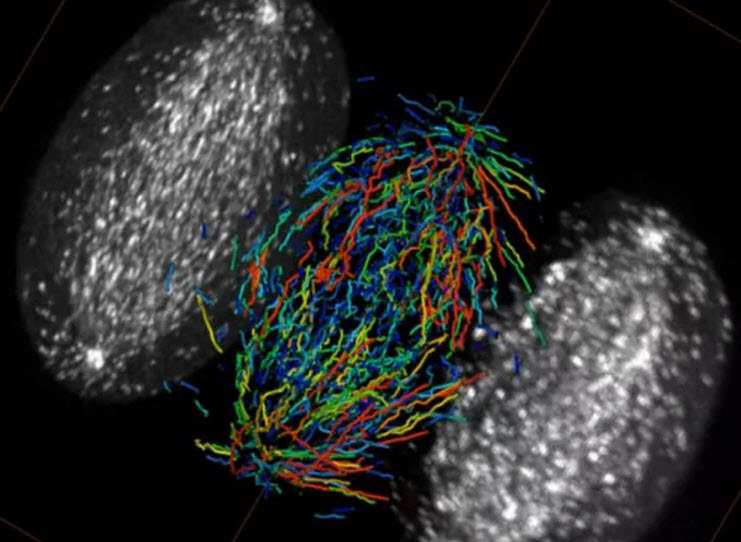
A single HeLa cell in metaphase (during mitosis), imaged by a lattice light sheet microscope. Growing microtubule endpoints and tracks are color coded by growth-phase lifetime (credit: Betzig Lab, HHMI/Janelia Research Campus, Mimori-Kiyosue Lab, RIKEN Center for Developmental Biology/Science)
A new imaging platform called a “lattice light sheet” developed by Nobel laureate Eric Betzig and colleagues at the Howard Hughes Medical Institute’s Janelia Research Campus is a significant leap forward for light microscopy. It captures high-resolution images rapidly and minimizes damage to cells, so it can image the three-dimensional activity of molecules, cells, and embryos in fine detail over longer periods than was previously possible, according to the HHMI scientists.
And that allows for stunning videos of biological processes across a range of sizes and time scales, from the movements of individual proteins to the development of entire animal embryos.
HHMI | Microtubule dynamics during mitosis in a Hela cell
The scientists, including postdoctoral researchers Bi-Chang Chen (now at the Research Center for Applied Sciences in Taiwan),Wesley Legant, and Kai Wang, described their new technology and its applications in the October 24, 2014, issue of the journal Science.
Betzig, one of three scientists sharing the 2014 Nobel Prize in Chemistry (for the development of super-resolved fluorescence microscopy), has developed a suite of new imaging technologies over the last ten years. The techniques have improved biologists’ ability to visually track the movements of cells’ tiniest structures — but there were always trade-offs. Imaging cells at high resolution in three dimensions usually meant sacrificing imaging speed, as well as subjecting cells to significant light-induced toxicity.
With the lattice light sheet, the Betzig team can now optimize their imaging technology for the questions that biologists want to answer.
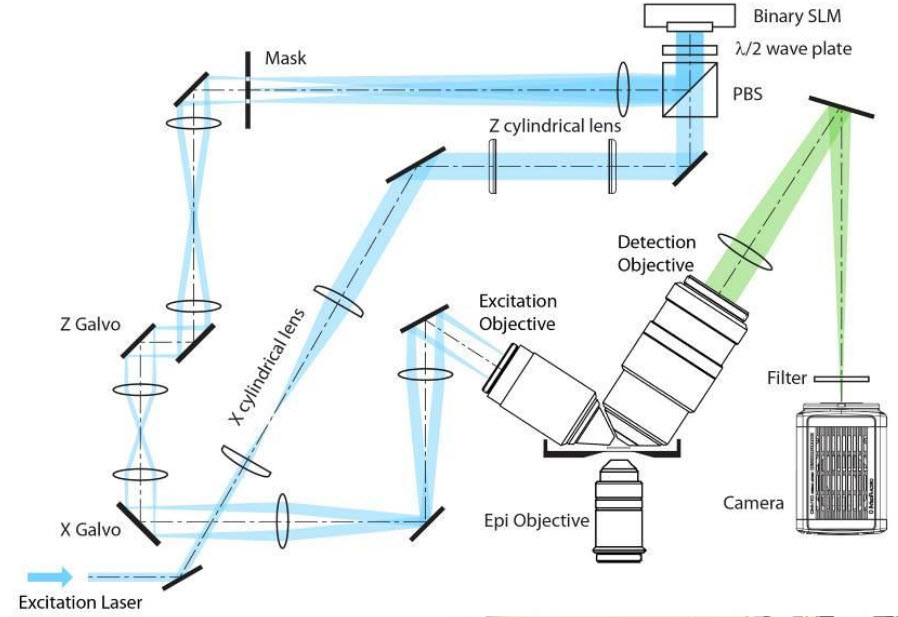
Schematic of the optical path through the lattice light sheet microscope (credit: HHMI/Science)
The new microscope evolved from one Betzig unveiled in 2011. The Bessel beam plane illumination microscope illuminates samples with a virtual sheet of light, created when a beam of non-diffracting light called a Bessel beam sweeps across the imaging field. It produces high-resolution images with less light damage than a traditional microscope, and is fast enough to record dynamic processes in living cells.
Betzig’s team had to incorporate a few tricks into that microscope to address a problem that arose from the shape of the Bessel beam. “It’s not just a thin pencil of light — it has these dimmer side lobes,” Betzig explains. “So when you sweep that across a sample, you get out-of-focus light.” One solution was to shift the beam step-by-step across the sample, illuminating it with a grating pattern. The researchers could then computationally strip out the blurriness caused by the Bessel beam’s side lobes – a technique known as structured illumination.
Structured illumination can also be used to overcome light microscopes’ usual limits of spatial resolution. To apply a super-resolution structured illumination technique developed at Janelia by the late Mats Gustafsson, Betzig’s team moved the Bessel beam to produce a lattice-like pattern of light. “With that we not only get rid of the side lobe stuff, we actually push the resolution a bit beyond the diffraction limit,” he says.
To reduce the time required to move the Bessel beam each time a sample was imaged, the developers split the beam into seven parallel parts, so each traveled just one-seventh of the original distance. Suddenly, the cells they were imaging seemed healthier.
“What was shocking to us was that by spreading the energy out across seven beams instead of one, the phototoxicity went way down,” Betzig says. “What I learned from that experience is that while the total dose of light you put on the cell is important, what’s far more important is the instantaneous power that you put on the cell.”
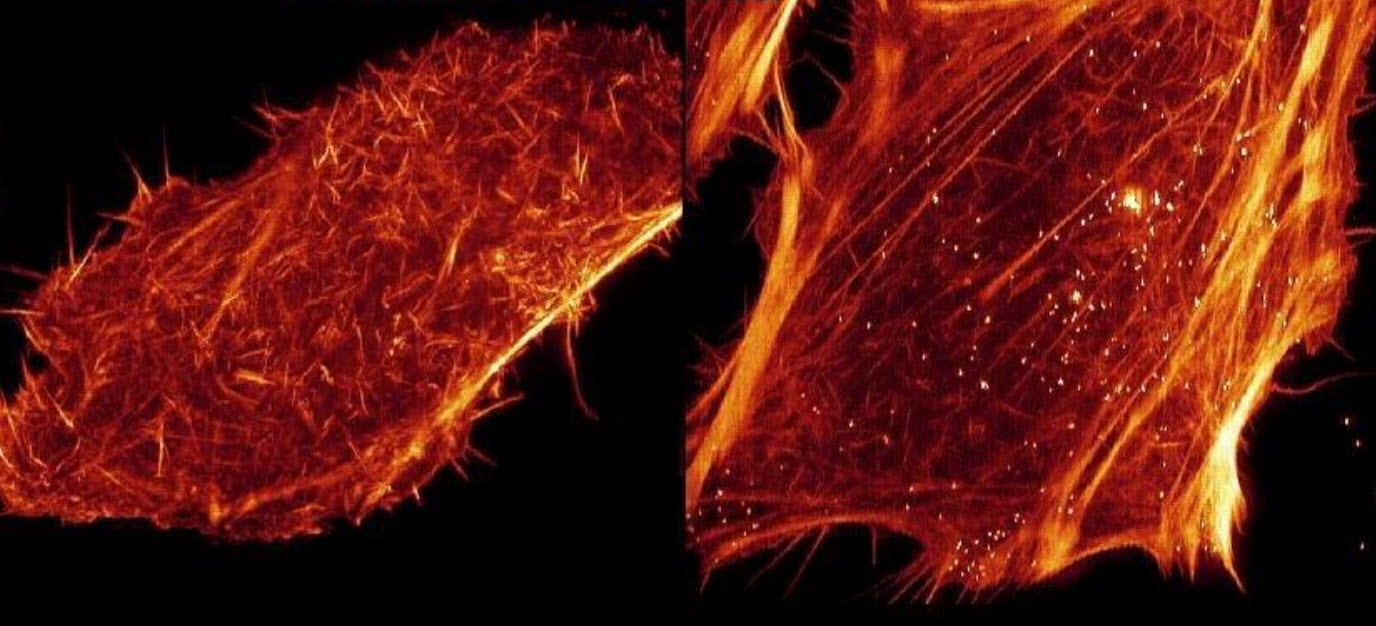
Renderings of HeLa cells expressing mEmerald-Lifeact, demonstrating the higher speed and reduced phototoxicity of the lattice light (right) compared to Bessel beam plane illumination (left) when each is applied to super-resolution structured illumination microscopy (SR-SIM) (credit: HHMI/Science)
The new microscope operates in two modes:
- The superresolution structured illumination microscopy (SIM) mode uses the principles of structured illumination to create very high-resolution images (150 nm x 280 nm in the xz plane). The final image is created by collecting and processing multiple images of every plane of the sample.
- In dithered mode, imaging can be sped up to capture faster processes, albeit at lower resolution (this is used in the video above). Light exposure, and thus damage to cells, is lower in the dithered mode.
Thirty teams of biologists have come to Janelia over the past year to find out what the lattice light sheet microscope can reveal about the systems they study. Chen, Legant, and Wang have worked with the researchers to optimize the technology for a variety of experiments. “This is not a single imaging technique,” Wang says. “It’s an imaging platform.”
Tracking single proteins in 3D
The microscope’s high resolution means it can track the movements of individual proteins in three dimensions, and Betzig says these applications are among the most exciting [see movie on “Single molecule tracking of TMR-labeled Sox2 transcription factors” below]. “Normally when people do single-molecule studies, they have to do them in thin, flat cells, because the out-of-focus light kills you if it’s thick.”
The lattice’s ultra-thin light sheet eliminates that problem, and permits single molecules to be seen even in large multicellular specimens. The microscope is also fast enough to track the rapid growth and retraction of cytoskeletal components in dividing cells (as in the video of microtubules above), and gentle enough to monitor the molecular dynamics of developmental processes that unfold over many hours.
The Science paper includes videos of these and other processes gleaned from the Betzig team’s collaborations, but the scientists say they represent only the beginning. “We know what the microscope can offer in terms of the imaging, but I think there are a lot of applications we haven’t even thought of yet,” Legant says.
Betzig wants the lattice light sheet to be widely used, even as technology development continues in his own lab. His team has built a second microscope for Janelia’s Advanced Imaging Center, where it will be available to visiting scientists free of charge, and deployed two more of the microscopes to labs at Harvard and the University of California, San Francisco. (Scientists who wish to use the lattice light sheet microscope for their own research can submit a proposal to Janelia’s Advanced Imaging Center at http://www.janelia.org/aic.)
In fact, Betzig’s team freely shares its designs, providing detailed instructions to scientists with the expertise to build their own version of the instrument. Zeiss has licensed the Bessel beam and lattice light sheet microscopy. “It takes a huge amount of effort to move from a successful high-tech prototype to broader adoption of an imaging technology,” Betzig says. “Ultimately, commercialization is the crucial last step to ensuring that these technologies can have broad impact in the research community.”
HHMI | Single molecule tracking of TMR-labeled Sox2 transcription factors in an ∼35 μm diameter spheroid of mouse embryonic stem cells, as seen by a dithered lattice light sheet at a fixed plane (top), a single Bessel beam scanned across the same plane (middle), and widefield, epi-illumination across the entire specimen (bottom).
UPDATE Oct. 24, 2014: Added movie: “Single molecule tracking of TMR-labeled Sox2 transcription factors” (bottom)
Abstract of Lattice Light Sheet Microscopy: Imaging Molecules, Cells, and Embryos at High Spatiotemporal Resolution
Although fluorescence microscopy provides a crucial window into the physiology of living specimens, many biological processes are too fragile, are too small, or occur too rapidly to see clearly with existing tools. We crafted ultrathin light sheets from two-dimensional optical lattices that allowed us to image three-dimensional (3D) dynamics for hundreds of volumes, often at subsecond intervals, at the diffraction limit and beyond. We applied this to systems spanning four orders of magnitude in space and time, including the diffusion of single transcription factor molecules in stem cell spheroids, the dynamic instability of mitotic microtubules, the immunological synapse, neutrophil motility in a 3D matrix, and embryogenesis in Caenorhabditis elegans and Drosophila melanogaster. The results provide a visceral reminder of the beauty and the complexity of living systems.