Using light to remotely trigger biochemical reactions
December 17, 2012
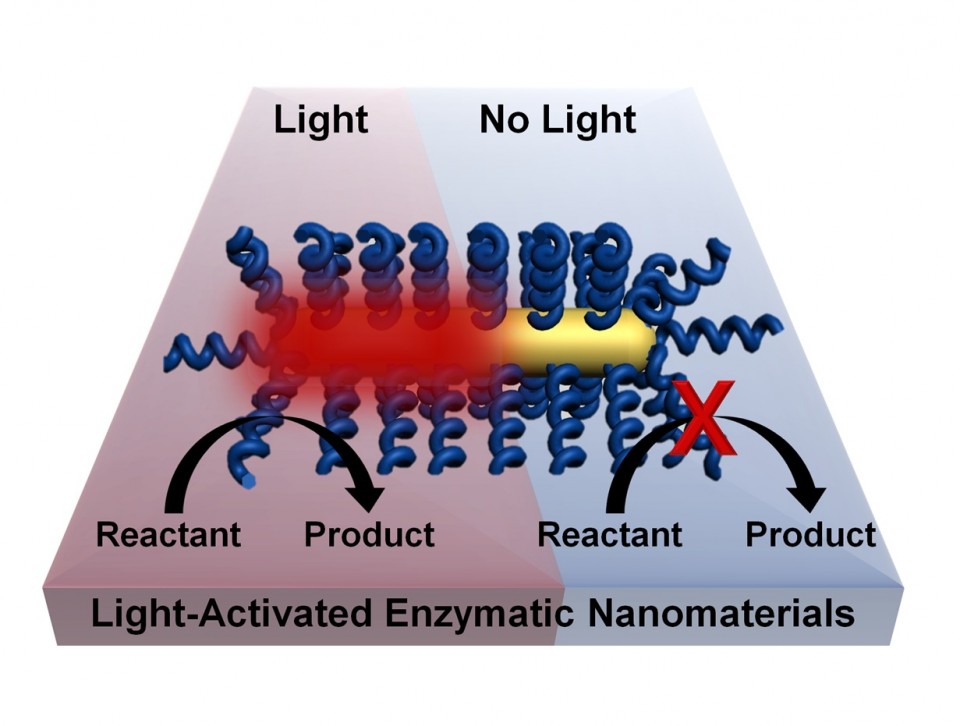
Chemical processes can be activated by light without the need for bulk heating of a material by coating nanorods with thermophilic enzymes that are activated at high temperatures. Lighting the plasmonic gold nanorod causes highly localized heating and activates the enzyme. (Credit: Lori Pretzer/Rice University)
A method for turning light into heat to trigger specific biochemical reactions remotely on demand has been developed by Rice University researchers. It uses materials derived from thermophile microbes, which thrive at high temperatures but shut down at room temperature.
The Rice project, led by postdoctoral fellow Matthew Blankschien and graduate student Lori Pretzer, combines enzymes from these creatures with plasmonic gold nanoparticles that heat up when exposed to near-infrared light. That activates the enzymes, which are then able to carry out their functions.
This effectively allows chemical processes to happen at lower temperatures. Because heating occurs only where needed — at the surface of the nanoparticle, where it activates the enzyme — the environment stays cooler.
Better industrial chemistry
The technique holds potential for industrial processes that now require heat or benefit from remote triggering with light.
“In the chemical industry, there’s always a need for better catalytic materials so they can run reactions more inexpensively, more ‘green’ and more sustainably,” said co-author Michael Wong, a Rice professor of chemical and biomolecular engineering and of chemistry. You shouldn’t run through gallons of solvent to make a milligram of product, even if you happen to be able to sell it for a lot of money.”
For industry, the potential energy savings alone may make the Rice process worth investigating. “Here we’re using ‘free’ energy,” Wong said. “Instead of needing a big boiler to produce steam, you turn on an energy-efficient light bulb, like an LED. Or open a window.”
Gold nanorods
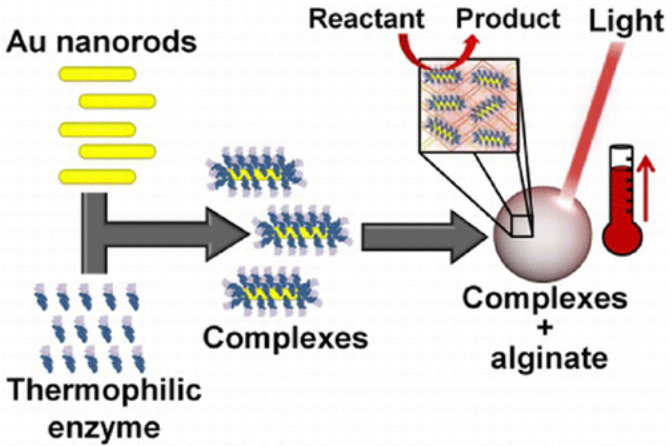
Light-induced activation of the thermophilic enzyme Aeropyrum pernix glucokinase, a key enzyme for the decomposition of glucose via the glycolysis pathway, increasing its rate of reaction 60% with light by conjugating the enzyme onto Au nanorods. The observed increase in enzyme activity corresponded to a local temperature increase within a calcium alginate encapsulate of 20 °C when compared to the bulk medium maintained at standard, nonthermophilic temperatures.
The particle at the center of the process is a gold nanorod about 10 nanometers wide and 30 long that heats up when hit with near-infrared light from a laser.
The rods are just the right size and shape to react to near-infrared light at around 800 nanometers. The light excites surface plasmons that ripple like water in a pool, in this case, emitting energy as heat.
Co-author Naomi Halas‘ Rice lab is famous for pioneering the use of gold nanoshells (a related material) to treat cancer by targeting tumors with particles that are bulk heated to kill tumors from the inside. The therapy is now in human trials.
The new research takes a somewhat different tack by heating nanoparticles draped with a model thermophilic enzyme, glucokinase, from Aeropyrum pernix.
A. pernix is a microbe discovered in 1996 thriving near hot underwater vents off the coast of Japan. At around 176 degrees Fahrenheit, A. pernix degrades glucose, a process necessary to nearly every living thing. The enzyme can be heated and cooled repeatedly.
Wong sees other possible uses for the new approach in the production of fuels from degradation of biomass like lignocellulose, for drug manufacture on demand — maybe from nanoparticle-infused tattoos on the body, or for lowering blood sugar concentrations as a different way to manage diabetes.
The research was supported by the Peter and Ruth Nicholas Postdoctoral Fellowship Program administered by the Richard E. Smalley Institute for Nanoscale Science and Technology, the Rice University Institute of Biosciences and Bioengineering Hamill Innovations Award Program, the Rice University Faculty Initiatives Fund, the Robert A. Welch Foundation, the National Security Science and Engineering Faculty Fellowship, the Defense Threat Reduction Agency, the Air Force Office of Scientific Research and the National Science Foundation.