Weighing molecules one at a time
August 28, 2012
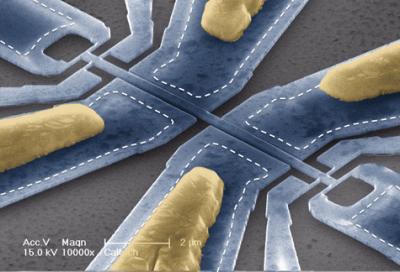
This scanning electron micrograph shows one of the molecule-weighing devices. The bridge-like section at the center vibrates sideways. The scale bar at the bottom is two microns. (Credit: Caltech/Scott Kelberg and Michael Roukes)
California Institute of Technology (Caltech) scientists have made the first-ever mechanical device that can measure the mass of individual molecules one at a time.
This new technology, the researchers say, will eventually help doctors diagnose diseases, enable biologists to study viruses and probe the molecular machinery of cells, and even allow scientists to better measure nanoparticles and air pollution.
The device — which is only a couple microns (millionths of a meter) in size — consists of a tiny, vibrating bridge-like structure. When a particle or molecule lands on the bridge, its mass changes the oscillating frequency in a way that reveals how much the particle weighs.
“As each particle comes in, we can measure its mass,” says Michael Roukes, the Robert M. Abbey Professor of Physics, Applied Physics, and Bioengineering at Caltech. “Nobody’s ever done this before.”
The new instrument is based on a technique Roukes and his colleagues developed over the last 12 years. In work published in 2009, they showed that a bridge-like device — called a nanoelectromechanical system (NEMS) resonator — could indeed measure the masses of individual particles, which were sprayed onto the apparatus. The difficulty, however, was that the measured shifts in frequencies depended not only on the particle’s actual mass, but also on where the particle landed. Without knowing the particle’s landing site, the researchers had to analyze measurements of about 500 identical particles in order to pinpoint its mass.
But with the new and improved technique, the scientists need only one particle to make a measurement. “The critical advance that we’ve made in this current work is that it now allows us to weigh molecules — one by one — as they come in,” Roukes says.
To do so, the researchers analyzed how a particle shifts the bridge’s vibrating frequency. All oscillatory motion is composed of vibrational modes. If the bridge just shook in the first mode, it would sway side to side, with the center of the structure moving the most. The second vibrational mode is at a higher frequency, in which half of the bridge moves sideways in one direction as the other half goes in the opposite direction, forming an oscillating S-shaped wave that spans the length of the bridge. There is a third mode, a fourth mode, and so on. Whenever the bridge oscillates, its motion can be described as a mixture of these vibrational modes.
The team found that by looking at how the first two modes change frequencies when a particle lands, they could determine the particle’s mass and position, explains Mehmet Selim Hanay, a postdoctoral researcher in Roukes’s lab and first author of the paper. “With each measurement we can determine the mass of the particle, which wasn’t possible in mechanical structures before.”
Measuring massive particles
Traditionally, molecules are weighed using a method called mass spectroscopy, in which tens of millions of molecules are ionized — so that they attain an electrical charge — and then interact with an electromagnetic field. By analyzing this interaction, scientists can deduce the mass of the molecules.
The problem with this method is that it does not work well for more massive particles — like proteins or viruses — which have a harder time gaining an electrical charge. As a result, their interactions with electromagnetic fields are too weak for the instrument to make sufficiently accurate measurements.
The new device, on the other hand, does work well for large particles. In fact, the researchers say, it can be integrated with existing commercial instruments to expand their capabilities, allowing them to measure a wider range of masses.
The researchers demonstrated how their new tool works by weighing a molecule called immunoglobulin M (IgM), an antibody produced by immune cells in the blood. By weighing each molecule — which can take on different structures with different masses in the body — the researchers were able to count and identify the various types of IgM. Not only was this the first time a biological molecule was weighed using a nanomechanical device, but the demonstration also served as a direct step toward biomedical applications.
Future instruments could be used to monitor a patient’s immune system or even diagnose immunological diseases. For example, a certain ratio of IgM molecules is a signature of a type of cancer called Waldenström macroglobulinemia.
In the more distant future, the new instrument could give biologists a view into the molecular machinery of a cell. Proteins drive nearly all of a cell’s functions, and their specific tasks depend on what sort of molecular structures attach to them — thereby adding more heft to the protein — during a process called posttranslational modification. By weighing each protein in a cell at various times, biologists would now be able to get a detailed snapshot of what each protein is doing at that particular moment in time.
Another advantage of the new device is that it is made using standard, semiconductor fabrication techniques, making it easy to mass-produce. That’s crucial, since instruments that are efficient enough for doctors or biologists to use will need arrays of hundreds to tens of thousands of these bridges working in parallel. “With the incorporation of the devices that are made by techniques for large-scale integration, we’re well on our way to creating such instruments,” Roukes says. This new technology, the researchers say, will enable the development of a new generation of mass-spectrometry instruments.
“This result demonstrates how the Alliance for Nanosystems VLSI, initiated in 2006, creates a favorable environment to carry out innovative experiments with state-of-the-art, mass-produced devices,” says Laurent Malier, the director of CEA-LETI. The Alliance for Nanosystems VLSI is the name of the partnership between Caltech’s Kavli Nanoscience Institute and CEA-LETI. “These devices,” he says,”will enable commercial applications, thanks to cost advantage and process repeatability.”
The team includes researchers from the Kavli Nanoscience Institute at Caltech and Commissariat à l’Energie Atomique et aux Energies Alternatives, Laboratoire d’électronique des technologies de l’information (CEA-LETI) in Grenoble, France.