Whole-brain imaging method identifies common brain disorders
July 22, 2016

Synaptic-density images of the human brain, derived from PET scans. The sequential images are coronal slices (from front to back of the brain), sagittal slices (from left to right), and transverse images (from bottom to top). (credit: Video by Yale PET Center)
How many of the estimated 100 trillion synapses in your brain are actually functioning? It’s an important question for diagnosis and treatment of people with common brain disorders, such as epilepsy, Alzheimer’s disease, autism, depression, schizophrenia, and traumatic brain injury (TBI), but one that could not be answered, except in an autopsy (or an invasive surgical sample of a small area).
Now a Yale-led team of researchers has developed a way to measure the density of synapses in the brain using a PET (positron emission tomography) scan. They invented a radioligand (a radioactive tracer that, when injected into the body, binds to a type of protein and “lights up” during a PET scan) called [11C]UCB-J that allows for imaging a protein (called SV2A) that is uniquely present in all synapses in the brain.
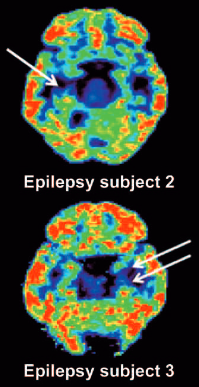
PET scan reveals unilateral mesial temporal sclerosis in epilepsy patients (white arrows indicate loss of [11C]UCB-J binding in the mesial temporal lobe). (credit: Sjoerd J. Finnema et al./Science Translational Medicine)
With this noninvasive method, researchers may now be able to follow the progression of many brain disorders by measuring changes in synaptic density over time or assess how well pharmaceuticals slow the loss of neurons.
Professor of radiology and biomedical imaging Richard Carson and his team plan future studies involving PET imaging of synapses for a variety of brain disorders.
Published July 20 in Science Translational Medicine, the study was supported in part by the Swebilius Foundation, UCB Pharma, and the National Center for Advancing Translational Science, a component of the National Institutes of Health.
Abstract of Imaging synaptic density in the living human brain
Chemical synapses are the predominant neuron-to-neuron contact in the central nervous system. Presynaptic boutons of neurons contain hundreds of vesicles filled with neurotransmitters, the diffusible signaling chemicals. Changes in the number of synapses are associated with numerous brain disorders, including Alzheimer’s disease and epilepsy. However, all current approaches for measuring synaptic density in humans require brain tissue from autopsy or surgical resection. We report the use of the synaptic vesicle glycoprotein 2A (SV2A) radioligand [11C]UCB-J combined with positron emission tomography (PET) to quantify synaptic density in the living human brain. Validation studies in a baboon confirmed that SV2A is an alternative synaptic density marker to synaptophysin. First-in-human PET studies demonstrated that [11C]UCB-J had excellent imaging properties. Finally, we confirmed that PET imaging of SV2A was sensitive to synaptic loss in patients with temporal lobe epilepsy. Thus, [11C]UCB-J PET imaging is a promising approach for in vivo quantification of synaptic density with several potential applications in diagnosis and therapeutic monitoring of neurological and psychiatric disorders.