Wireless device delivers drugs to brain and triggers neurons via remote control
July 16, 2015
Tiny, implantable devices are capable of delivering light or drugs to specific areas of the brain, potentially improving drug delivery to targeted regions of the brain and reducing side effects. Eventually, the devices may be used to treat pain, depression, epilepsy and other neurological disorders in people. (credit: Alex David Jerez Roman)
A team of researchers has developed a tiny “wireless optofluidic neural probe” the width of a human hair that can be implanted in the brain and triggered by remote control to deliver drugs and activate targeted populations of brain cells.
The technology, demonstrated for the first time in mice, may one day be used to treat pain, depression, epilepsy, and other neurological disorders in people by targeting therapies to specific brain circuits with fewer side effects, according to the researchers at Washington University School of Medicine in St. Louis and the University of Illinois at Urbana-Champaign.
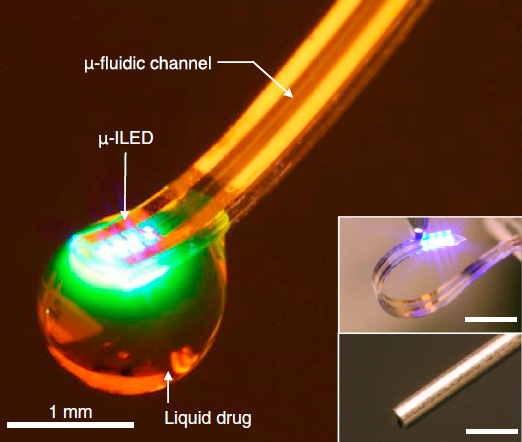
Soft optofluidic neural probe during simultaneous drug delivery and photostimulation (from micro-ILED). Drugs would be delivered via the fluidic channel and activated with light as needed. (Insets) Comparison of such a device (top) and a conventional metal cannula (bottom). Scale bars, 1 mm. (credit: Jae-Woong Jeong et al./Cell)
The research builds on earlier work in optogenetics, a technology that makes individual brain cells sensitive to light and then activates those targeted populations of cells with flashes of light.
The study was published online today (July 16) in the journal Cell and will appear in the July 30 print issue.
Previous attempts to deliver drugs or other agents, such as enzymes or other compounds, to experimental animals have required the animals to be tethered to rigid pumps and tubes that restricted their movement and often caused them to experience stress.
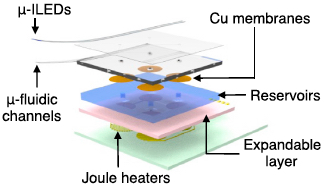
Exploded-view schematic diagram that illustrates an array of inorganic light-emitting diodes mounted on top of a soft microfluidic system that includes four separate microfluidic channels, each connected to a set of fluid reservoirs that include copper membranes as hermetic seals, expandable composite materials as mechanical transducers, and microscale heating elements as actuators (credit: Jae-Woong Jeong et al./Cell)
The new wireless optofluidic neural probes were built with four chambers to carry drugs directly into the brain via microfluidic channels and microscale pumps, and the probes are soft like brain tissue.
New tool for mapping brain-circuit activity
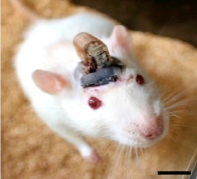
A freely moving rat with head-mounted device for drug delivery and photostimulation via the optofluidic system. The device is remotely controlled via infrared technology, similar to that used in a TV remote. Scale bar, 1 cm. (credit: Jae-Woong Jeong et al./Cell)
By activating brain cells with drugs and with light, the scientists are getting an unprecedented look at the inner workings of the brain.
“This is the kind of revolutionary tool development that neuroscientists need to map out brain circuit activity,” said James Gnadt, PhD, program director at the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NIH).
“It’s very much in line with the goals of the NIH’s BRAIN Initiative, a program designed to accelerate the development and application of new technologies to shed light on the complex links between brain function and behavior.”
The new devices may ultimately also help people with neurological disorders and other problems, according to co-first author Jae-Woong Jeong, PhD, a former postdoctoral researcher at the University of Illinois and now assistant professor of electrical, computer and energy engineering at the University of Colorado, Boulder.
“The device can remain in the brain and function for a long time without causing inflammation or neural damage,” Jeong said.
The researchers also believe that similar, more flexible devices could have applications in areas of the body other than the brain, including peripheral organs.
Messing with mice minds
As part of the study, the researchers, who clearly are having way too much fun, showed that by delivering a drug to one side of an animal’s brain, they could stimulate neurons involved in movement, which caused the mouse to move in a circle.
In other mice, shining a light directly onto brain cells expressing a light-sensitive protein prompted the release of dopamine, a neurotransmitter that rewarded the mice by making them feel good. The mice then returned to the same location in a maze to seek another reward. But the researchers were able to interfere with that light-activated pursuit by remotely controlling the release of a drug that blocks the action of dopamine on its receptors.
The researchers hope to incorporate a design much like a printer’s ink cartridge so that drugs can continue to be delivered to specific cells in the brain, or elsewhere in the body, for as long as required and needed to replace the entire device.
Abstract of Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics
In vivo pharmacology and optogenetics hold tremendous promise for dissection of neural circuits, cellular signaling, and manipulating neurophysiological systems in awake, behaving animals. Existing neural interface technologies, such as metal cannulas connected to external drug supplies for pharmacological infusions and tethered fiber optics for optogenetics, are not ideal for minimally invasive, untethered studies on freely behaving animals. Here, we introduce wireless optofluidic neural probes that combine ultrathin, soft microfluidic drug delivery with cellular-scale inorganic light-emitting diode (m-ILED) arrays. These probes are orders of magnitude smaller than cannulas and allow wireless, programmed spatiotemporal control of fluid delivery and photostimulation. We demonstrate these devices in freely moving animals to modify gene expression, deliver peptide ligands, and provide concurrent photostimulation with antagonist drug delivery to manipulate mesoaccumbens rewardrelated behavior. The minimally invasive operation of these probes forecasts utility in other organ systems and species, with potential for broad application in biomedical science, engineering, and medicine.