Wireless power system replaces batteries in implants
September 5, 2012
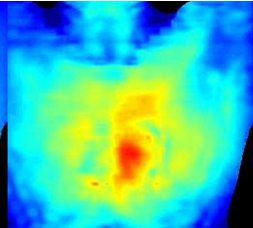
Wireless power transmission to a device in the human heart. Red indicates greatest power; blue is least. (Credit: John Ho, Stanford Engineering)
Stanford University engineers have demonstrated the feasibility of a super-small, implantable cardiac device that gets its power from radio waves transmitted from a small transmitter on the surface of the body.
This is an impressive achievement that may lead to replacing bulky batteries in implants. That means the implants can be further miniaturized, while eliminating surgery to replace or charge batteries (or require a wired connection outside the body).
Devices that might be improved include permanent pacemakers, cochlear implants, brain stimulators, drug pumps, spinal cord stimulators, and swallowable endoscopes (“pillcams”) that travel the digestive tract — virtually any medical applications where device size and power matter.
The receiver device (a cube 0.8 millimeter on a side) was implanted 5 centimeters inside the chest on the surface of the heart — a depth once thought out of reach for wireless power transmission. Power is transmitted from a coil located outside the chest.
Last year, Ada Poon, assistant professor of electrical engineering, who headed up the research, demonstrated a wirelessly powered, self-propelled device capable of swimming through the bloodstream.
Miniaturizing implants
Her latest device works by a combination of inductive (used in wirelessly charging batteries) and radiative transmission of power. Both are types of electromagnetic transfer in which a transmitter sends radio waves to a coil of wire inside the body. The radio waves induce an electrical current in the coil sufficient to operate a small device.
These is an inverse relationship between frequency and coil size: using a high frequency transmitter allows for smaller coils. So for implantable medical devices, the goal is a high-frequency transmitter, but existing mathematical models have held that high-frequency radio waves do not penetrate far enough into human tissue, necessitating the use of low-frequency transmitters and large antennas, which are impractical for implantable devices.
Poon got around this limitation by using alternating electric and magnetic fields. According to their revised models, the researchers found that the maximum power transfer through human tissue occurs at about 1.7GHz (close to cell phone frequencies), much higher than previously thought, allowing for increasing power transfer by about 10 times over earlier devices and shrinking the receiving antenna by a factor of 10 as well, making wireless implantable devices feasible.
The engineers estimated that a millimeter-radius coil is capable of harvesting more than 50 microwatts of power, well in excess of the needs of a recently demonstrated 8-microwatt pacemaker.
Engineering challenges
However, electronic medical devices must meet stringent health standards established by the IEEE (Institute of Electrical and Electronics Engineers), particularly with regard to tissue heating. Second, the team found that the receiving and transmitting antennas had to be optimally oriented to achieve maximum efficiency. Differences in alignment of just a few degrees could produce troubling drops in power.
“As the human heart and body are in constant motion, solving this issue was critical to the success of our research,” said Poon. The team responded by designing an innovative slotted transmitting antenna structure that delivers consistent power efficiency regardless of orientation of the two antennas.
The new design serves additionally to focus the radio waves precisely at the point inside the body where the implanted device rests on the surface of the heart — increasing the electric field where it is needed most, but canceling it elsewhere. This helps reduce overall tissue heating to levels well within the IEEE standards. Poon has applied for a patent on the antenna structure.
This research was made possible by funding from the C2S2 Focus Center, one of six research centers funded under the Focus Center Research Program, a Semiconductor Research Corporation entity.