3D neural reconstruction guided with biocompatible nanofiber scaffolds and hydrogels
April 3, 2015
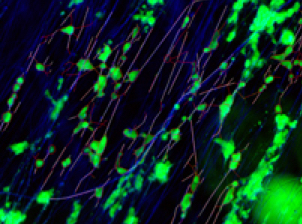
Images of neuronal cultures in 3D hydrogel scaffold (green) with laminin-coated nanofibers (blue) and growing neurites (red) (credit: Richard J. McMurtrey/Journal of Neural Engineering)
Damage to neural tissue is typically permanent and causes lasting disability in patients. But a method for reconstructing neural tissue using patterned nanofibers in 3D hydrogel structures promises to one day help in the restoration of functional neuroanatomical pathways and structures at sites of spinal cord injury, traumatic brain injury, tumor resection, stroke, and neurodegenerative diseases.
Richard J. McMurtrey, director of the Institute of Neural Regeneration & Tissue Engineering and affiliated with the University of Oxford, found that neurite outgrowth from neurons in the hydrogel followed the nanofiber scaffolding, particularly when the nanofibers were coated with a type of cell adhesion molecule called laminin. The coated nanofibers also enhanced the length of growing neurites.
“The direction and pattern of neurite outgrowth can be guided and controlled using relatively simple combinations of structural cues and biochemical signaling factors,” he said.
The method is an alternative to one developed by Penn State researchers involving transformation of glial cells into functioning neural cells, as described on KurzweilAI on Wednesday April 1.
Reconstructing brain tissue
The next step will be replicating more complex structures using a patient’s own induced stem cells to reconstruct damaged or diseased sites in the nervous system. “Neural stem cells hold incredible potential for restoring damaged cells in the nervous system, and 3D reconstruction of neural tissue is essential for replicating the complex anatomical structure and function of the brain and spinal cord,” said McMurtrey, author an open-access paper in the Journal of Neural Engineering.
According to McMurtrey, these 3D reconstructions can then be used to implant into the damaged areas of neural tissue to help reconstruct specific neuroanatomical structures and integrate with the proper neural circuitry in order to restore function.
Successful restoration of function would require training of the new neural circuitry over time, but by selecting the proper neurons and forming them into native architecture, implanted neural stem cells would have a much higher chance of providing successful outcomes.
The scaffolding and hydrogel materials are biocompatible and biodegradable, and the hydrogels can also help to maintain the microstructure of implanted cells and prevent them from washing away in the cerebrospinal fluid that surrounds the brain and spinal cord.
“Great potential” for regenerative medicine
McMurtrey also noted that by making these site-specific reconstructions of neural tissue, researchers can also make models for studying disease mechanisms and developmental processes just by using skin cells that are induced into pluripotent stem cells and into neurons from patients with a variety of diseases and conditions.
“The 3D constructs enable a realistic replication of the innate cellular environment and also enable study of diseased human neurons without needing to biopsy neurons from affected patients and without needing to make animal models that can fail to replicate the full array of features seen in humans,” said McMurtrey.
The ability to engineer neural tissue from stem cells and biomaterials holds great potential for regenerative medicine, he added. “The combination of stem cells, functionalized hydrogel architecture, and patterned and functionalized nanofiber scaffolding enables the formation of unique 3D tissue constructs, and these engineered constructs offer important applications in brain and spinal cord tissue that has been damaged by trauma, stroke, or degeneration.”
Institute of Neural Regeneration & Tissue Engineering | Restoring Structure and Function of 3D Neural Tissue
Abstract of Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control
OBJECTIVE: Neural tissue engineering holds incredible potential to restore functional capabilities to damaged neural tissue. It was hypothesized that patterned and functionalized nanofiber scaffolds could control neurite direction and enhance neurite outgrowth.
APPROACH: A method of creating aligned electrospun nanofibers was implemented and fiber characteristics were analyzed using environmental scanning electron microscopy. Nanofibers were composed of polycaprolactone (PCL) polymer, PCL mixed with gelatin, or PCL with a laminin coating. Three-dimensional hydrogels were then integrated with embedded aligned nanofibers to support neuronal cell cultures. Microscopic images were captured at high-resolution in single and multi-focal planes with eGFP-expressing neuronal SH-SY5Y cells in a fluorescent channel and nanofiber scaffolding in another channel. Neuronal morphology and neurite tracking of nanofibers were then analyzed in detail.
MAIN RESULTS: Aligned nanofibers were shown to enable significant control over the direction of neurite outgrowth in both two-dimensional (2D) and three-dimensional (3D) neuronal cultures. Laminin-functionalized nanofibers in 3D hyaluronic acid (HA) hydrogels enabled significant alignment of neurites with nanofibers, enabled significant neurite tracking of nanofibers, and significantly increased the distance over which neurites could extend. Specifically, the average length of neurites per cell in 3D HA constructs with laminin-functionalized nanofibers increased by 66% compared to the same laminin fibers on 2D laminin surfaces, increased by 59% compared to 2D laminin-coated surface without fibers, and increased by 1052% compared to HA constructs without fibers. Laminin functionalization of fibers also doubled average neurite length over plain PCL fibers in the same 3D HA constructs. In addition, neurites also demonstrated tracking directly along the fibers, with 66% of neurite lengths directly tracking laminin-coated fibers in 3D HA constructs, which was a 65% relative increase in neurite tracking compared to plain PCL fibers in the same 3D HA constructs and a 213% relative increase over laminin-coated fibers on 2D laminin-coated surfaces.
SIGNIFICANCE: This work demonstrates the ability to create unique 3D neural tissue constructs using a combined system of hydrogel and nanofiber scaffolding. Importantly, patterned and biofunctionalized nanofiber scaffolds that can control direction and increase length of neurite outgrowth in three-dimensions hold much potential for neural tissue engineering. This approach offers advancements in the development of implantable neural tissue constructs that enable control of neural development and reproduction of neuroanatomical pathways, with the ultimate goal being the achievement of functional neural regeneration.