A battery alternative to costly, rare lithium
October 13, 2015
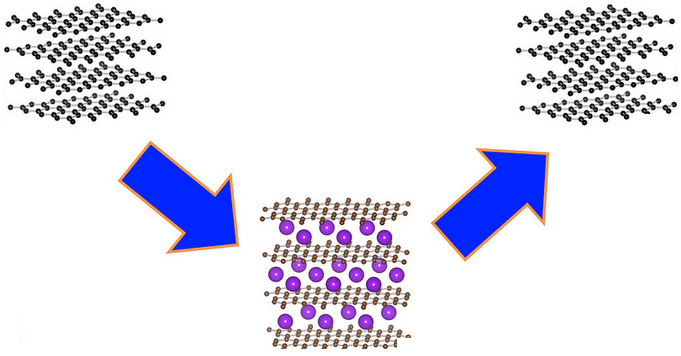
Potassium ions (purple) are compatible with graphite electrodes (black) and can function in a charge-discharge cycle, chemists have now shown (credit: Oregon State University)
Overturning nearly a century of a scientific dogma, Oregon State University chemists have now shown that potassium could potentially replace rare, costly lithium in a new potassium-ion battery.
“For decades, people have assumed that potassium couldn’t work with graphite or other bulk carbon anodes in a battery,” said Xiulei (David) Ji, the lead author of the study and an assistant professor of chemistry in the College of Science at Oregon State University. “That assumption is incorrect,” he said. “It’s really shocking that no one ever reported on this issue for 83 years.”
The findings are important, the researchers say, because they open some new alternatives for batteries that can work with well-established, inexpensive graphite as the anode (the high-energy reservoir of electrons).
Lithium is quite rare, found in only 0.0017 percent, by weight, of the Earth’s crust. Because of that, it’s comparatively expensive, and also difficult to recycle.
Cost, availability problems with lithium
“The cost-related problems with lithium are sufficient that you won’t really gain much with economies of scale,” Ji said. “With most products, as you make more of them, the cost goes down. With lithium the reverse may be true in the near future. So we have to find alternatives.”
That alternative, he said, may be potassium, which is 880 times more abundant in the Earth’s crust than lithium. The new findings show that it can work effectively with graphite or soft carbon in the anode of an electrochemical battery.
“It’s safe to say that the energy density (amount of electrical power per unit volume) of a potassium-ion battery may never exceed that of lithium-ion batteries,” he said. But potassium-ion batteries may provide a “long cycling life, a high power density [ability to discharge quickly], a lot lower cost, and be ready to take the advantage of the existing manufacturing processes of carbon anode materials.”
Electrical energy storage in batteries is essential not only for consumer products such as cell phones and computers, but also in transportation, industry power backup, micro-grid storage, and for the wider use of renewable energy.
The Journal of the American Chemical Society published the findings from this discovery, which was supported by the U.S. Department of Energy. A patent is pending on the new technology.
Abstract of Carbon Electrodes for K-Ion Batteries
We for the first time report electrochemical potassium insertion in graphite in a nonaqueous electrolyte, which can exhibit a high reversible capacity of 273 mAh/g. Ex situ XRD studies confirm that KC36, KC24, and KC8 sequentially form upon potassiation, whereas depotassiation recovers graphite through phase transformations in an opposite sequence. Graphite shows moderate rate capability and relatively fast capacity fading. To improve the performance of carbon K-ion anodes, we synthesized a nongraphitic soft carbon that exhibits cyclability and rate capability much superior to that of graphite. This work may open up a new paradigm toward rechargeable K-ion batteries.