A battery you never have to replace
April 21, 2016
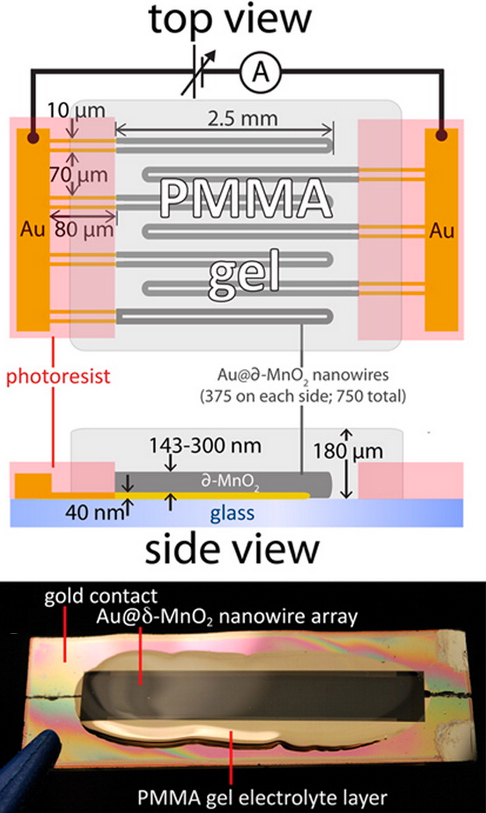
Top: Schematic diagram of all-nanowire-based capacitor (similar to a battery), using gold-manganese dioxide conductors and PMMA gel layer. Bottom: photograph of the capacitor containing 750 parallel nanowire loops patterned onto a glass microscope slide. (credit: Mya Le Thai/ACS Energy Lett.)
University of California, Irvine researchers have invented a new nanowire-based battery material that can be recharged hundreds of thousands of times, moving us closer to a battery that would never require replacement.
It could lead to commercial batteries with greatly lengthened lifespans for computers, smartphones, appliances, cars, and spacecraft.
The design is based on nanowires, which are highly conductive and feature a large surface area for the storage and transfer of electrons.
Currently, nanowires are extremely fragile and don’t hold up well to repeated discharging and recharging (cycling). In a typical lithium-ion battery, they expand and grow brittle, which leads to cracking.
UCI researchers have solved this problem by coating a gold nanowire in a manganese dioxide shell and encasing the assembly in an electrolyte made of a Plexiglas-like gel. The liquid electrolyte is replaced with a poly(methyl methacrylate) (PMMA) gel electrolyte. The combination is reliable and resistant to failure.
The study leader, UCI doctoral candidate Mya Le Thai, cycled the testing electrode up to 200,000 times over three months without detecting any loss of capacity or power and without fracturing any nanowires. The findings were published Wednesday Apr. 20 in an open-access paper in the American Chemical Society’s Energy Letters.
“Mya was playing around, and she coated this whole thing with a very thin gel layer and started to cycle it,” said senior author Reginald Penner, chair of UCI’s chemistry department. “She discovered that just by using this gel, she could cycle it hundreds of thousands of times without losing any capacity. That was crazy, because these things typically die in dramatic fashion after 5,000 or 6,000 or 7,000 cycles at most.”
The researchers think the gel plasticizes the metal oxide in the battery and gives it flexibility, preventing cracking.
“The coated electrode holds its shape much better, making it a more reliable option,” Thai said. “This research proves that a nanowire-based battery electrode can have a long lifetime and that we can make these kinds of batteries a reality.”
Abstract of 100k Cycles and Beyond: Extraordinary Cycle Stability for MnO2 Nanowires Imparted by a Gel Electrolyte
We demonstrate reversible cycle stability for up to 200 000 cycles with 94–96% average Coulombic efficiency for symmetrical δ-MnO2 nanowire capacitors operating across a 1.2 V voltage window in a poly(methyl methacrylate) (PMMA) gel electrolyte. The nanowires investigated here have a Au@δ-MnO2 core@shell architecture in which a central gold nanowire current collector is surrounded by an electrodeposited layer of δ-MnO2 that has a thickness of between 143 and 300 nm. Identical capacitors operating in the absence of PMMA (propylene carbonate (PC), 1.0 M LiClO4) show dramatically reduced cycle stabilities ranging from 2000 to 8000 cycles. In the liquid PC electrolyte, the δ-MnO2 shell fractures, delaminates, and separates from the gold nanowire current collector. These deleterious processes are not observed in the PMMA electrolyte.