A biocompatible, transparent therapeutic window to the brain
July 14, 2016
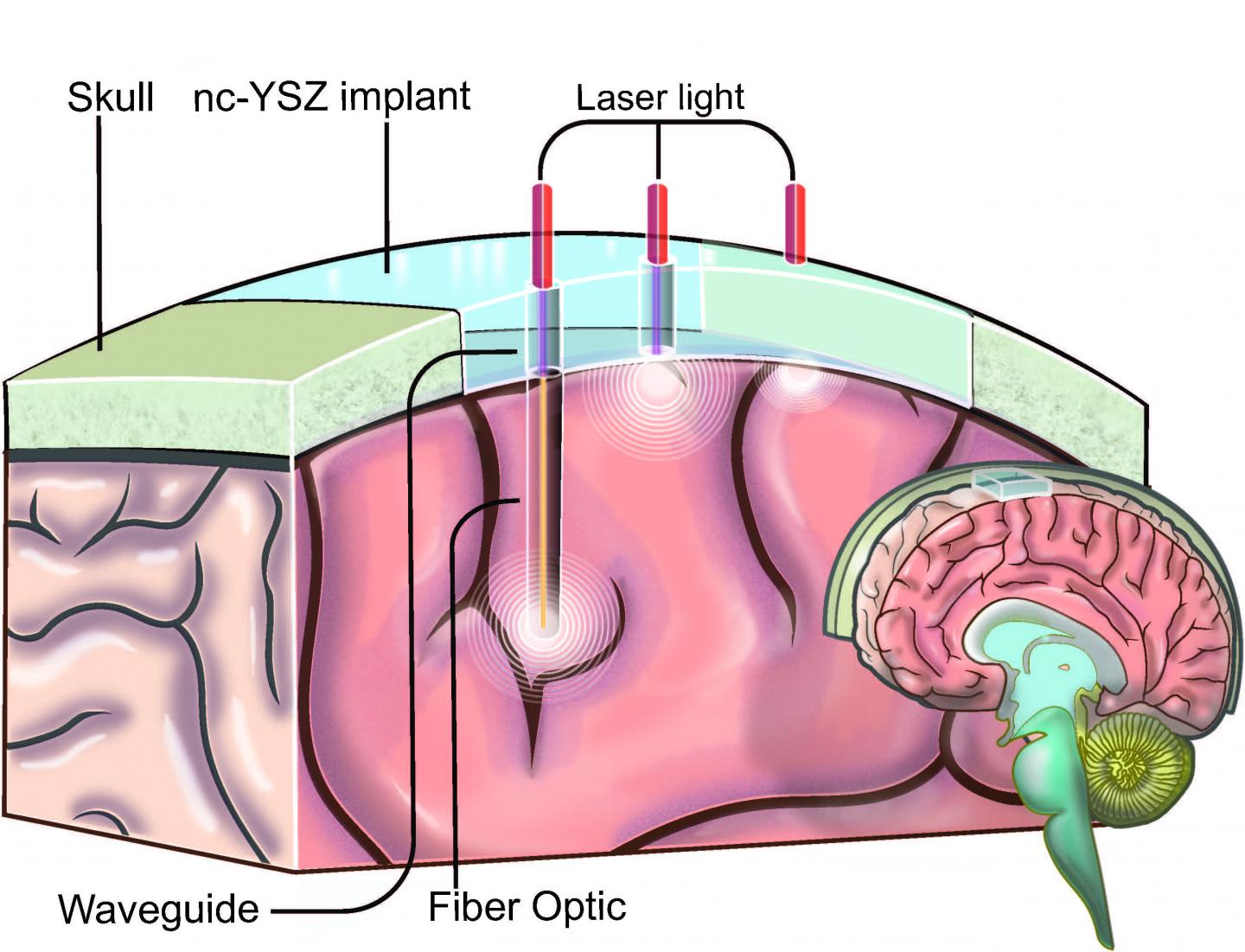
An illustration showing how the “window to the brain” transparent skull implant created by UC Riverside researchers would work (credit: UC Riverside)
Researchers at the University of California, Riverside have developed a transparent “window to the brain” — a skull implant that is biocompatible, infection-resistant, and does not need to be repetitively replaced.
Part of the ongoing “Window to the Brain” project, a multi-institution, cross-disciplinary effort, the idea is to use transparent skull implants to provide laser diagnosis and treatment of a wide variety of brain pathologies, including brain cancers, traumatic brain injury, stroke, and neurodegenerative diseases, without requiring repeated craniotomies (a surgical operation in which a bone flap is temporarily removed from the skull to access the brain). Such operations are vulnerable to bacterial infections.
A biocompatible transparent material
The researchers have developed a transparent version of the material yttria-stabilized zirconia (YSZ), a ceramic material used in hip implants and dental crowns.
The researchers implanted the material in a hamster, where it integrated into the host tissue without causing an immune response or other adverse effects, as they describe in a paper in the journal Nanomedicine: Nanotechnology, Biology and Medicine. The internal toughness of YSZ, which is more impact-resistant and biocompatible than the titanium, thermoplastic polymers, and glass-based materials developed by other researchers, makes it “the only transparent skull implant that could conceivably be used in humans,” according to the researchers.
Treating bacterial infections
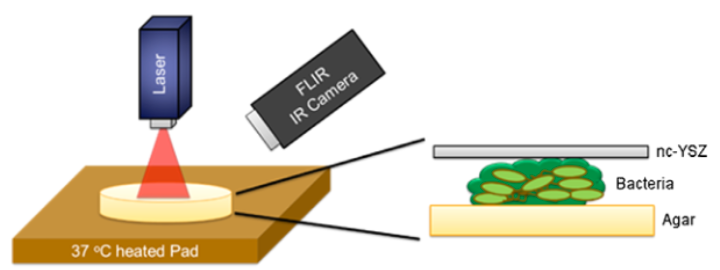
Schematic diagram showing treatment of biofilm formation with near-infrared laser light via a transparent YSZ material, monitored by a thermal IR camera (credit: Yasaman Damestani et al./Lasers in Surgery and Medicine)
The scientists also developed a way to use the same laser light used in brain treatments to treat incidental bacterial infections. In a lab study, described in a paper in the journal Lasers in Surgery and Medicine, the researchers treated E. Coli infections by aiming laser light through the transparent implant, without having to remove the implant and without causing an immune response or other adverse effects to surrounding tissue.
“This was an important finding because it showed that the combination of our transparent implant and laser-based therapies enables us to treat not only brain disorders, but also to tackle bacterial infections that are common after cranial implants. These infections are especially challenging to treat because many antibiotics do not penetrate the blood brain barrier,” said Devin Binder, M.D., a neurosurgeon and neuroscientist in UCR’s School of Medicine and a collaborator on the project.
The Window to the Brain project is a multi-institution, interdisciplinary partnership led by Guillermo Aguilar, professor of mechanical engineering in UCR’s Bourns College of Engineering, and Santiago Camacho-López, from the Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE) in Mexico.
Last October, the team received $3.6 million from the National Science Foundation’s Partnerships in International Research and Education (PIRE) program, which pairs U.S. universities with others around the world. An additional $1 million was from Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico’s entity in charge of promoting scientific and technological activities. The remainder of the money came from in-kind contributions from the Mexican universities.
The team’s long-term goal is to see the technology become the standard of care for patients with brain disorders.
Abstract of Inflammatory response to implantation of transparent nanocrystalline yttria-stabilized zirconia using a dorsal window chamber model
The long-range goal of the windows to the brain (WttB) is to improve patient care by providing a technique for delivery and/or collection of light into/from the brain, on demand, over large areas, and on a chronically-recurring basis without the need for repeated craniotomies. To evaluate the potential of nanocrystalline yttria-stabilized-zirconia (nc-YSZ) cranial implant for optical therapy and imaging, in vivo biocompatibility was studied using the dorsal window chamber model in comparison with control (no implant) and commercially available cranial implant materials (PEEK and PEKK). The host tissue response to implant was characterized by using transillumination and fluorescent microscopy to measure leukocyte adhesion, blood vessel diameter, blood flow rate, and vascular permeability over two weeks. The results indicated the lack of inflammatory reaction of the host tissue to nc-YSZ at the microscopic level, suggesting that nc-YSZ is a good alternative material for cranial implants.
Abstract of Evaluation of laser bacterial anti-fouling of transparent nanocrystalline yttria-stabilized-zirconia cranial implant
Background and Objective: The development and feasibility of a novel nanocrystalline yttria-stabilized-zirconia (nc-YSZ) cranial implant has been recently established. The purpose of what we now call “window to the brain (WttB)” implant (or platform), is to improve patient care by providing a technique for delivery and/or collection of light into/from the brain, on demand, over large areas, and on a chronically recurring basis without the need for repeated craniotomies. WttB holds the transformative potential for enhancing light-based diagnosis and treatment of a wide variety of brain pathologies including cerebral edema, traumatic brain injury, stroke, glioma, and neurodegenerative diseases. However, bacterial adhesion to the cranial implant is the leading factor for biofilm formation (fouling), infection, and treatment failure. Escherichia coli (E. coli), in particular, is the most common isolate in gram-negative bacillary meningitis after cranial surgery or trauma. The transparency of our WttB implant may provide a unique opportunity for non-invasive treatment of bacterial infection under the implant using medical lasers.
Study Design/Materials and Methods: A drop of a diluted overnight culture of BL21-293 E. coli expressing luciferase was seeded between the nc-YSZ implant and the agar plate. This was followed by immediate irradiation with selected laser. After each laser treatment the nc-YSZ was removed, and cultures were incubated for 24 hours at 37 °C. The study examined continuous wave (CW) and pulsed wave (PW) modes of near-infrared (NIR) 810 nm laser wavelength with a power output ranging from 1 to 3 W. During irradiation, the temperature distribution of nc-YSZ surface was monitored using an infrared thermal camera. Relative luminescence unit (RLU) was used to evaluate the viability of bacteria after the NIR laser treatment.
Results: Analysis of RLU suggests that the viability of E. coli biofilm formation was reduced with NIR laser treatment when compared to the control group (P < 0.01) and loss of viability depends on both laser fluence and operation mode (CW or PW). The results demonstrate that while CW laser reduces the biofilm formation more than PW laser with the same power, the higher surface temperature of the implant generated by CW laser limits its medical efficacy. In contrast, with the right parameters, PW laser produces a more moderate photothermal effect which can be equally effective at controlling bacterial growth.
Conclusions: Our results show that E. coli biofilm formation across the thickness of the nc-YSZ implant can be disrupted using NIR laser treatment. The results of this in vitro study suggest that using nc-YSZ as a cranial implant in vivo may also allow for locally selective, non-invasive, chronic treatment of bacterial layers (fouling) that might form under cranial implants, without causing adverse thermal damage to the underlying host tissue when appropriate laser parameters are used. Lasers Surg. Med. © 2016 Wiley Periodicals, Inc.