A biosensor that’s 1 million times more sensitive
March 29, 2016
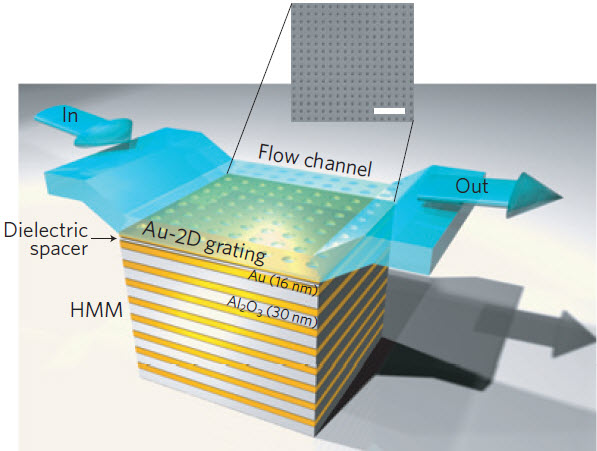
A schematic representation of the miniaturized gold-aluminum oxide hyperbolic metamaterial (HMM) sensor device with a fluid flow channel, showing a scanning electron microscope (SEM) image [gray inset] of the 2D subwavelength gold diffraction grating on top of the hyperbolic metamaterials layers (scale bar, 2 µm) (credit: Kandammathe Valiyaveedu Sreekanth et al./Nature Materials)
An optical sensor that’s 1 million times more sensitive than the current best available has been developed by Case Western Reserve University researchers. Based on nanostructured metamaterials, it can identify a single lightweight molecule in a highly dilute solution.
The research goal is to provide oncologists a way to detect a single molecule of an enzyme produced by circulating cancer cells. That could allow doctors to diagnose and monitor patients with certain cancers far earlier than possible today.
“The prognosis of many cancers depends on the stage of the cancer at diagnosis,” said Giuseppe “Pino” Strangi, professor of physics at Case Western Reserve and research leader. “Very early, most circulating tumor cells express proteins of a very low molecular weight, less than 500 Daltons,” Strangi explained. “These proteins are usually too small and in too low a concentration to detect with current test methods, yielding false negative results.
“With this platform, we’ve detected proteins of 244 Daltons, which should enable doctors to detect cancers earlier — we don’t know how much earlier yet,” he said. “This biosensing platform may help to unlock the next era of initial cancer detection.”
The researchers believe the sensing technology will also be useful in diagnosing and monitoring other diseases.
A biological sieve
The nanosensor, which fits in the palm of a hand, acts like a biological sieve, capable of isolating a small protein molecule weighing less than 800 quadrillionths of a nanogram from an extremely dilute solution.
To make the device so sensitive, Strangi’s team faced two long-standing barriers: Light waves cannot detect objects smaller than their own physical dimensions (about 500 nanometers, depending on wavelength). And molecules in dilute solutions float in Brownian (random) motion and are unlikely to land on the sensor’s surface.
The solution was to use a microfluidic channel to restrict the molecules’ ability to float around and a plasmon-based metamaterial made of 16 nanostructured layers of reflective and conductive gold and transparent aluminum oxide, a dielectric, each 10s of atoms thick. Light directed onto and through the layers is concentrated into a very small volume much smaller than the wavelength of light.*
“It’s extremely sensitive,” Strangi said. “When a small molecule lands on the surface, it results in a large local modification, causing the light to shift.” Depending on the size of the molecule, the reflecting light shifts different amounts. The researchers hope to learn to identify specific biomarker and other molecules for different cancers by their light shifts.
To add specificity to the sensor, the team added a layer of trap molecules — molecules that bind specifically with the molecules they hunt. In tests, the researchers used two trap molecules to catch two different biomolecules: bovine serum albumin, with a molecular weight of 66,430 Daltons, and biotin, with a molecular weight of 244 Daltons. Each produced a signature light shift.
Other researchers have reported using plasmon-based biosensors to detect biotin in solutions at concentrations ranging from more than 100 micromoles per liter to 10 micromoles per liter. This device proved 1 million times more sensitive, finding and identifying biotin at a concentration of 10 picomoles per liter.
Testing and clinical use in process
Strangi’s lab is working with other oncologists worldwide to test the device and begin moving the sensor toward clinical use.
In Cleveland, Strangi and Nima Sharifi, MD, co-leader of the Genitourinary Cancer Program for the Case Comprehensive Cancer Center, have begun testing the sensor with proteins related to prostate cancers.
“For some cancers, such as colorectal and pancreatic cancer, early detection is essential,” said Sharifi, who is also the Kendrick Family Chair for Prostate Cancer Research at Cleveland Clinic. “High sensitivity detection of cancer-specific proteins in blood should enable detection of tumors when they are at an earlier disease stage.
“This new sensing technology may help us not only detect cancers, but what subset of cancer, what’s driving its growth and spread, and what it’s sensitive to,” he said. “The sensor, for example, may help us determine markers of aggressive prostate cancers, which require treatments, or indolent forms that don’t.”
The research is published online in the journal Nature Materials.
* The top gold layer is perforated with holes, creating a grating that diffuses light shone on the surface into two dimensions. The incoming light, which is several hundreds of nanometers in wavelength, appears to be confined and concentrated in a few nanometers at the interface between the gold and the dielectric layer. As the light strikes the sensing area, it excites free electrons causing them to oscillate and generate a highly confined propagating surface wave, called a surface plasmon polariton. This propagating surface wave will in turn excite a bulk wave propagating across the sensing platform. The presence of the waves cause deep sharp dips in the spectrum of reflecting light. The combination and the interplay of surface plasmon and bulk plasmon waves are what make the sensor so sensitive. Strangi said. By exciting these waves through the eight bilayers of the metamaterial, they create remarkably sharp resonant modes. Extremely sharp and sensitive resonances can be used to detect smaller objects.
Abstract of Extreme sensitivity biosensing platform based on hyperbolic metamaterials
Optical sensor technology offers significant opportunities in the field of medical research and clinical diagnostics, particularly for the detection of small numbers of molecules in highly diluted solutions. Several methods have been developed for this purpose, including label-free plasmonic biosensors based on metamaterials. However, the detection of lower-molecular-weight (<500 Da) biomolecules in highly diluted solutions is still a challenging issue owing to their lower polarizability. In this context, we have developed a miniaturized plasmonic biosensor platform based on a hyperbolic metamaterial that can support highly confined bulk plasmon guided modes over a broad wavelength range from visible to near infrared. By exciting these modes using a grating-coupling technique, we achieved different extreme sensitivity modes with a maximum of 30,000 nm per refractive index unit (RIU) and a record figure of merit (FOM) of 590. We report the ability of the metamaterial platform to detect ultralow-molecular-weight (244 Da) biomolecules at picomolar concentrations using a standard affinity model streptavidin–biotin.