A ‘DNA nanotrain’ for targeted cancer drug transport
May 2, 2013
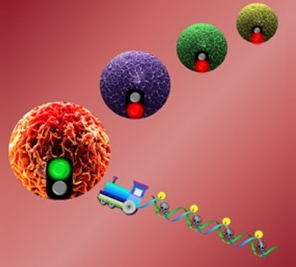
Chemotherapy drugs are specifically transported to target cancer cells carried on DNA “box cars” and powered by an aptamer “locomotive,” unloaded, and then induce cytotoxicity to cancer cells (credit: University of Florida)
University of Florida researchers have developed a “DNA nanotrain” that fast-tracks its payload of cancer-fighting drugs and bioimaging agents to tumor cells deep within the body.
The nanotrains can cost-effectively deliver high doses of drugs to precisely targeted cancers and other medical maladies without leaving behind toxic nano-clutter.
DNA nanotechnology holds great promise as a new way to deliver chemotherapy directly to cancer cells, but until now, scientists have not been able to direct nanotherapies to consistently differentiate cancer cells from healthy ones. Other limiting factors include high costs, too-small amounts of drugs delivered, and potential toxic side effects.
“Most nanotechnology relies on a nanoparticle approach, and the particles are made of inorganic materials; after they’ve been used as a carrier for the drug, they’ll be left inside the body,” said the study’s lead investigator, Weihong Tan, a UF distinguished professor of chemistry, professor of physiology and functional genomics, and a member of the UF Shands Cancer Center and the UF Genetics Institute.
“Compared to existing nanostructures, our nanotrain is easier and cheaper to make, is highly specific to cancer cells, has a lot of drug-loading power, and is very much biocompatible.”
Tan’s DNA nanotrain is a three-dimensional structure composed of short strands of DNA tethered together into one long train. On the end of the nanotrain is an aptamer, a tiny piece of nucleic acid serving as the train’s “locomotive” on biochemical autopilot to home in on and bind to specific cancer cells. Trailing behind are tethered DNA structures that serve as side-by-side, high-capacity “box cars,” transporting bioimaging agents or drug cargos to their targets.
“The beauty of the nanotrain is that by using different disease biomarkers, you can hitch different types of DNA probes as the train’s ‘locomotive’ to recognize and target different types of cancers,” Tan said. “We’ve precisely targeted leukemia, lung and liver cancer cells, and because the DNA probes are so precise in targeting only specific types of cancer cells, we’ve seen dramatic reduction in drug toxicity in comparison to standard chemotherapies, which don’t discriminate well between cancerous and healthy cells.”
Tan and his colleagues report that the DNA nanotrains can be cost-effectively made by mixing bits of DNA in a liquid medium. The mixture is then exposed to a compound that stimulates the pieces of DNA to seek each other out and self-assemble into the DNA nanotrains. The type of cancer cell the DNA nanotrain will seek out and destroy is determined by the specific compound added to the mixture as the trigger.
The study demonstrated in vitro and in mice that the DNA nanotrains exclusively target the cancer cells for which their probes were programmed. The DNA probes go straight to the cancer cells, leading the nanotrains to dock on the cell membranes and gain entry into the cells. Once inside, the drug payloads disperse, killing the cancer cells, a process Tan and his team monitored in real time by measuring the amount of fluorescent light emitted. The biodegradable components of the DNA nanotrains decay with the dead cancer cells and are removed by the body’s normal housekeeping mechanisms.
“Our study found that when loaded with anticancer drugs, these nanotrains inhibited tumor growth in mice more than in those that received drugs injected freely into the bloodstream. What’s more exciting is that the mice treated with these nanotrains suffered dramatically fewer side effects than those treated with free drugs,” said Guizhi Zhu, a UF doctoral student who was instrumental in the study. “This is what we aim to achieve for future clinical health care of cancer patients.”
In addition to longer survival and inhibited tumor growth, the mice that were treated with nanotrain drug delivery experienced less weight loss and are in better condition physically than both the mice that received injected therapy and the mouse control group that received no treatment. Tan and his team attribute these improved outcomes to greatly reduced toxicity achieved by the targeted nanotrain drug delivery.
“We think we have demonstrated that these DNA nanotrains are a promising targeted drug transport platform for delivery of cancer chemotherapeutics with very low toxicity to healthy tissues, and that the platform has wide application for many different cancer types,” Tan said. “Moving forward, we are working to identify optimum dosage using mouse models for T-cell leukemia, lung and liver cancers and triple negative breast cancer.
“It’s very exciting, but we still have a long way to go before human trials,” he said.