A new way to beam power to medical chips deep inside the body
May 20, 2014
A Stanford electrical engineer has invented a way to wirelessly transfer power deep inside the body, and use this power to run tiny electronic medical gadgets such as pacemakers, nerve stimulators, or new sensors and devices yet to be developed.
The discoveries reported Monday May 19 in the Proceedings of the National Academy of Sciences (PNAS) culminate years of efforts by Ada Poon, an assistant professor of electrical engineering, to eliminate the bulky batteries and clumsy recharging systems that prevent medical devices from being more widely used.
The technology could provide a path toward a new type of medicine that allows physicians to treat diseases with electronics rather than drugs. “We need to make these devices as small as possible to more easily implant them deep in the body and create new ways to treat illness and alleviate pain,” said Poon.
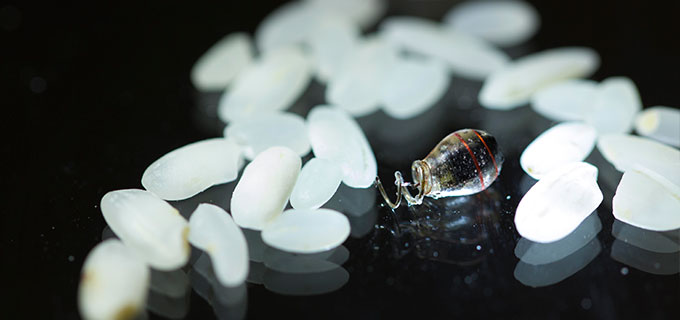
The PNAS paper describes how Poon’s team built an electronic device smaller than a grain of rice that acts as a pacemaker. It can be powered or recharged wirelessly by holding a power source about the size of a credit card above the device, outside of the body.
New generation of sensors and stimulators possible
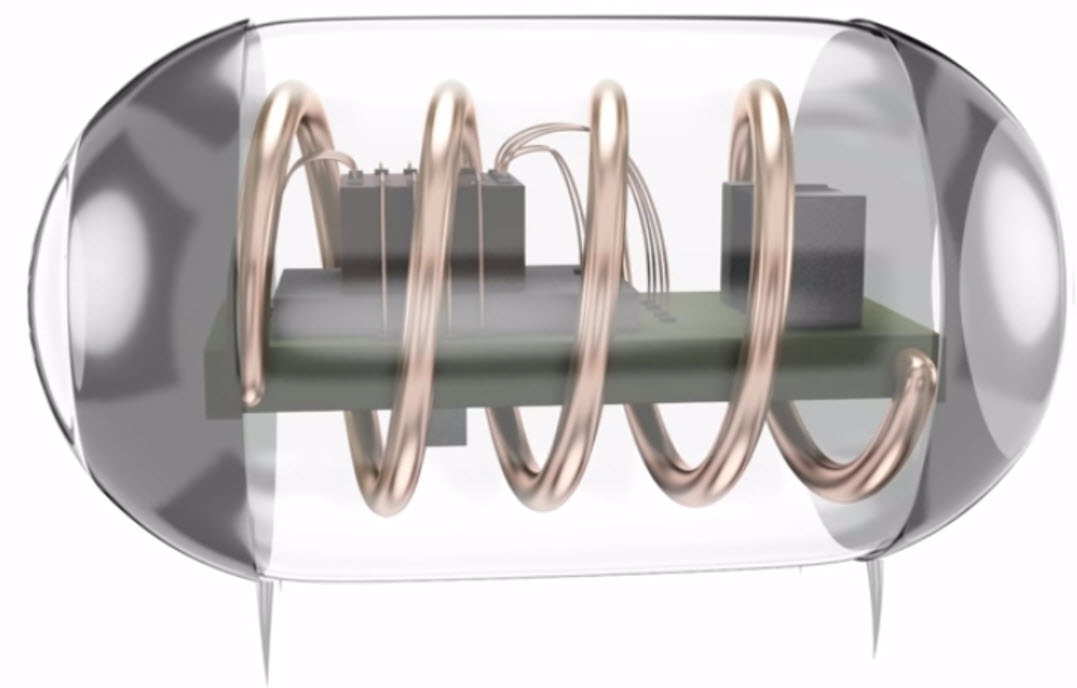
Illustration of a batteryless miniaturized implant with power-harvesting coil, rectifier, integrated circuit, and electrodes, all contained in an implantable device the size of a grain of rice (credit: Austin Yee)
The central discovery is an engineering breakthrough that creates a new type of wireless power transfer that can safely penetrate deep inside the body (more than 5 centimeters), using roughly the same power as a cell phone. Poon says an independent laboratory that tests cell phones found that her system fell well below the exposure levels for human safety.
Her lab has tested this wireless charging system in a pig and used it to power a tiny pacemaker in a rabbit. She is currently preparing the system for testing in humans. Should such tests be approved and prove successful, it would still take several years to satisfy the safety and efficacy requirements for using this wireless charging system in commercial medical devices.
Poon believes this discovery will spawn a new generation of programmable microimplants: sensors to monitor vital functions deep inside the body, electrostimulators to change neural signals in the brain, and drug delivery systems to apply medicines directly to affected areas.
Stanford electrical engineer Ada Poon has invented a way to wirelessly transfer power deep inside the body. The technology could provide a path toward new medical devices.
Alternatives to drug therapies
William Newsome, director of the Stanford Neurosciences Institute, said Poon’s work created the potential to develop “electroceutical” treatments as alternatives to drug therapies.
Newsome, who was not involved in Poon’s experiments but is familiar with her work, said such treatments could be more effective than drugs for some disorders because electroceutical approaches would implant devices to directly modulate activity in specific brain circuits. Drugs, by comparison, act globally throughout the brain.
“To make electroceuticals practical, devices must be miniaturized, and ways must be found to power them wirelessly, deep in the brain, many centimeters from the surface,” said Newsome, the Harman Family Provostial Professor of Neurobiology at Stanford, adding: “The Poon lab has solved a significant piece of the puzzle for safely powering implantable microdevices, paving the way for new innovation in this field.”
How it works
The PNAS paper describes the work of an interdisciplinary research team including John Ho and Alexander Yeh, electrical engineering graduate students in Poon’s lab; Yuji Tanabe, a visiting scholar, and Ramin Beygui, M.D., an Associate Professor of Cardiothoracic Surgery at the Stanford University Medical Center.
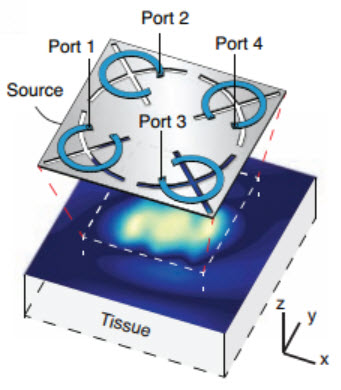
The crux of the discovery involves a new way to control electromagnetic waves inside the body.
Before Poon’s discovery, there was a clear divide between the two main types of electromagnetic waves in everyday use, called far-field and near-field waves.
Far-field waves, like those broadcast from radio towers, can travel over long distances. But when they encounter biological tissue, they either reflect off the body harmlessly or get absorbed by the skin as heat. Either way, far-field electromagnetic waves have been ignored as a potential wireless power source for medical devices.
Near-field waves can be safely used in wireless power systems. Some current medical devices like hearing implants use near-field technology. But they can only transfer power over short distances, which tends to keep such devices close to the skin and limits their usefulness deep inside the body.
‘Mid-field wireless transfer’
What Poon did was to blend the safety of near-field waves with the reach of far-field waves. She accomplished this by taking advantage of a simple fact: waves travel differently when they come into contact with different materials such as air, water or biological tissue.
With this principle in mind, Poon designed a power source using a patterned metal plate that generated a special type of near-field wave. When this special wave moved from air to skin, it changed its characteristics in a way that enabled it to propagate in the body. She called this new method “mid-field wireless transfer.”
In the PNAS experiment, Poon used her midfield transfer system to send power directly to tiny medical implants. But it is also possible to build tiny batteries into microimplants, and then recharge these batteries wirelessly using the midfield system. This is not possible with today’s technologies.
“With this method, we can safely transmit power to tiny implants in organs like the heart or brain, well beyond the range of current near-field systems,” said Ho, a graduate student in Poon’s lab and co-author on the paper.
Abstract of Proceedings of the National Academy of Sciences paper
The ability to implant electronic systems in the human body has led to many medical advances. Progress in semiconductor technology paved the way for devices at the scale of a millimeter or less (“microimplants”), but the miniaturization of the power source remains challenging. Although wireless powering has been demonstrated, energy transfer beyond superficial depths in tissue has so far been limited by large coils (at least a centimeter in diameter) unsuitable for a microimplant. Here, we show that this limitation can be overcome by a method, termed midfield powering, to create a high-energy density region deep in tissue inside of which the power-harvesting structure can be made extremely small. Unlike conventional near-field (inductively coupled) coils, for which coupling is limited by exponential field decay, a patterned metal plate is used to induce spatially confined and adaptive energy transport through propagating modes in tissue. We use this method to power a microimplant (2 mm, 70 mg) capable of closed-chest wireless control of the heart that is orders of magnitude smaller than conventional pacemakers. With exposure levels below human safety thresholds, milliwatt levels of power can be transferred to a deep-tissue (>5 cm) microimplant for both complex electronic function and physiological stimulation. The approach developed here should enable new generations of implantable systems that can be integrated into the body at minimal cost and risk.